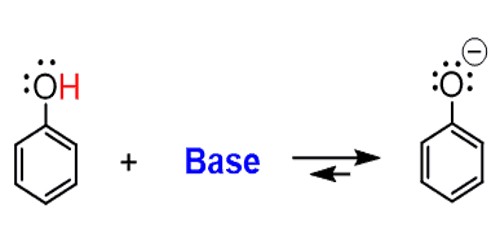
A base is a substance that reacts with an acid in an acid-base reaction. It is a substance that can accept a hydrogen ion (H+) from another substance. A chemical can accept a proton if it has a negative charge, or if the molecule has an electronegative atom like oxygen, nitrogen, or chlorine that is rich in electrons. A base is the opposite of an acid. Like acids, some bases are strong and others are weak. The weak bases are less likely to accept protons, while the strong bases quickly take protons in solution or from other molecules. An acid is a base’s “chemical opposite”. An acid is a substance that will donate a hydrogen atom to the base. Bases differ from acids in their potential for accepting rather than releasing hydrogen ions. Types of bases include Arrhenius base, Bronsted-Lowry base, and Lewis base.
Bases have a pH greater than 7.0. Weak bases generally have a pH value of 7–9 while strong bases have a pH value of 9–14. Examples of bases include hydroxides and soap.
How bases work
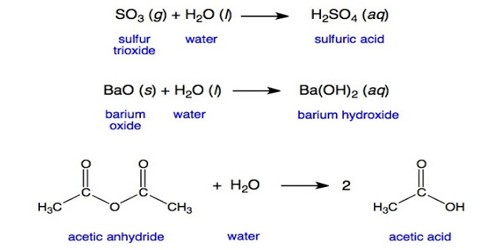
Bases can be used to neutralize acids. When a base, often OH–, accepts a proton from acid, it forms a water molecule that is harmless. When all of the acids and bases react to form water molecules and other neutral salts, it is called neutralization. Acids can also be used to neutralize bases.
Every base has a conjugate acid formed by adding a hydrogen atom to the base. For example, NH3 (ammonia) is a base and its conjugate acid is the ammonium ion, NH4+. A weak base forms a strong conjugate acid and a strong base forms a weaker conjugate acid. Since ammonia is a moderately strong base, ammonium is a considerably weaker acid.
Characteristics
Chemicals were bases due to their observable properties. In this respect, bases were substances that tasted bitter, were slippery and turned litmus dye from red to blue. Bases have these characteristics:
- Bitter taste (opposed to the sour taste of acids)
- Slimy, or soapy feel on fingers (Slippery)
- Strong bases may react violently with acids. An acid spill can be safely neutralized by using a mild base.
- Bases turn red litmus paper blue
- Bases are substances that contain metal oxides or hydroxides
- Bases which are soluble in water form alkalis (soluble bases)
Properties of a Base
A base displays several characteristic properties:
- Strong bases and concentrated bases are caustic. They react vigorously with acids and organic matter.
- Bases react in predictable ways with pH indicators. A base turns litmus paper blue, methyl orange-yellow, and phenolphthalein pink.
- A basic solution has a pH greater than 7.
Uses:
Some common household products are bases. For example, caustic soda and drain cleaner are made from sodium hydroxide, a strong base. Ammonia or an ammonia-based cleaner such as windows and glass cleaner is basic. These stronger bases may cause skin irritation. Other bases, like cooking ingredients sodium bicarbonate (baking soda) or cream of tartar, are basic, but these are not harmful and suitable for cooking.
Safety
Gloves should always be worn when handling bases. If skin irritation is encountered, the affected area should be rinsed thoroughly with cold water. If that does not stop the problem, contact medical help as soon as possible.
Strong bases
A strong base is a base that gives off a hydroxide ion, OH–, when putting in water. There are eight of them.
- Lithium hydroxide-LiOH
- Sodium hydroxide-NaOH
- Potassium hydroxide-KOH
- Rubidium hydroxide-RbOH
- Cesium hydroxide-CsOH
- Calcium hydroxide-Ca(OH)2
- Strontium hydroxide-Sr(OH)2
- Barium hydroxide-Ba(OH)2
Information Source: