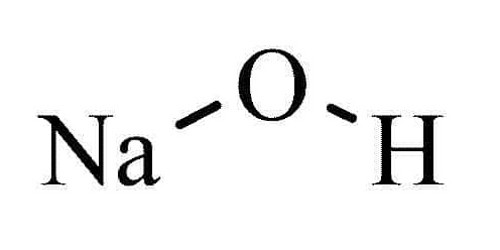Sodium hydroxide is an alkali which is also known as caustic soda. Caustic means “burning” and caustic soda takes its name from the way it can burn the skin. It is a popular strong base used in the industry. It has the chemical formula of NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.
It is a base, meaning it has a high pH. It is the most common thing used to raise the pH of solutions; for example, to neutralize an acid. It is a highly versatile substance used in a variety of manufacturing processes.
Properties
As a solid, the alkali is deliquescent and readily absorbs moisture and carbon dioxide from the air. It dissolves easily in water and makes the water warm when this happens.
- Chemical formula: NaOH
- Molar mass: 39.9971 g mol−1
- Appearance: White, waxy, opaque crystals
- Odor: odorless
- Density: 2.13 g/cm3
- Melting point: 323 °C (613 °F; 596 K)
- Boiling point: 1,388 °C (2,530 °F; 1,661 K)
- Solubility in water: 418 g/L (0 °C); 1000 g/L (25 °C); 3370 g/L (100 °C)
- Solubility: soluble in glycerol; negligible in ammonia; insoluble in ether; slowly soluble in propylene glycol
- Solubility in methanol:238 g/L
- Solubility in ethanol: <<139 g/L
- Vapor pressure: <2.4 kPa (at 20 °C)
Making sodium hydroxide
Sodium hydroxide can be made (with chlorine and hydrogen) using the chloralkali process. A solution of sodium chloride is electrolyzed and sodium hydroxide is made around the cathode, where water is reduced to hydrogen gas and hydroxide ion. The hydrogen is released and the hydroxide bonds with sodium to make sodium hydroxide. Special care is required to prepare a solution of sodium hydroxide or NaOH in water because considerable heat is liberated by the exothermic reaction. The solution may splatter or boil.
2Na+ (from salt) + 2H2O → 2NaOH + H2 at cathode
2 Cl– (from salt) → Cl2 at the anode
Uses:
Sodium hydroxide is used as a solution called lye to make soap. It is used in many scenarios where it is desirable to increase the alkalinity of a mixture or to neutralize acids. Lye is also used to unclog drains. Its dissolving properties and its ability to easily break surface tension are fundamental for its uses. It works in two ways. First, it combines with grease to make soap. Second, it dissolves hair (which is soluble in any basic solution).
It is used to manufacture many everyday products, such as paper, aluminum, commercial drain and oven cleaners, and soap and detergents.