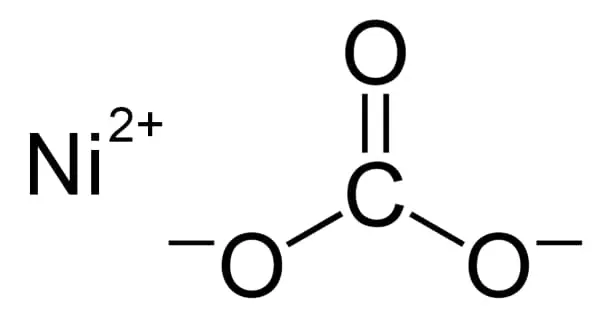
Nickel(II) carbonate refers to one or more inorganic compounds that contain nickel and carbonate. It is a crystalline inorganic compound that, when heated, emits toxic gases. The most important nickel carbonate from an industrial standpoint is basic nickel carbonate, which has the formula Ni4CO3(OH)6(H2O)4. NiCO3 and its hexahydrate are simpler carbonates that are more likely to be encountered in the laboratory. All are green paramagnetic solids containing Ni2+ cations.
Nickel carbonate is used in electroplating, the preparation of nickel monoxide, the production of colored glass, and as a catalyst in wastewater treatment. Exposure to this substance can result in severe dermatitis, skin allergies, and asthma-like symptoms, as well as effects on the lungs, kidneys, gastrointestinal tract, and neurological system.
Properties
Nickel carbonate is a light green crystalline substance, which is almost insoluble (0.093 g/L) in water (25°C), nonsoluble in hot water, and soluble in acids. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in the electroplating of nickel.
- Molecular Weight: 304.116
- Appearance: Green powder
- Melting point: decomposes
- Density: 4.390
- Form: Solid
- Color: Light Green
- Water Solubility: Soluble in dilute acids. Insoluble in cold water

Preparation
When calcium carbonate is heated in a sealed tube with a nickel chloride solution at 150°C, anhydrous nickel carbonate precipitates. Alternatively, treating nickel powder with ammonia and carbon dioxide, then boiling off the ammonia, produces pure carbonate.
When sodium carbonate is added to a solution of Ni(II) salts, impure basic nickel carbonate precipitates.
Structure and reactions
NiCO3 adopts a structure like calcite, consisting of nickel in an octahedral coordination geometry.
Nickel carbonates are hydrolyzed upon contact with aqueous acids to give solutions containing the ion [Ni(H2O)6]2+, liberating water and carbon dioxide in the process. Calcining (heating to drive offCO2 and water) of these carbonates gives nickel oxide:
NiCO3 → NiO + CO2
The nature of the resulting oxide depends on the nature of the precursor. The oxide obtained from the basic carbonate is often most useful for catalysis. Basic nickel carbonate can be made by treating solutions of nickel sulfate with sodium carbonate:
4 Ni2+ + CO32- + 6 OH– + 4 H2O → Ni4CO3(OH)6(H2O)4
The hydrated carbonate has been prepared by electrolytic oxidation of nickel in the presence of carbon dioxide:
Ni + O + CO2 + 6 H2O → NiCO3(H2O)4
Uses
Nickel carbonates are used in some ceramic applications and as catalyst precursors. Sulphamate baths, metal phosphating, electroplating, and ceramic applications all make use of it. It is used as a catalyst precursor and as an intermediate in the hydrometallurgical purification of nickel from its ores.
It is used to make nickel catalysts and a variety of nickel specialty compounds. It is also used as a neutralizer in nickel plating solutions. Other applications include glass coloring and the production of ceramic pigments.
Natural occurrence
The natural nickel carbonate is known as gaspéite – a rare mineral. Basic Ni carbonates also have some natural representatives.