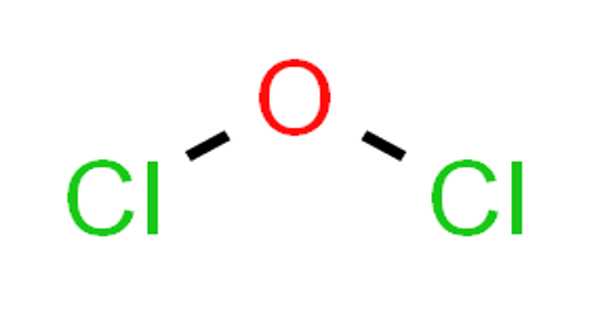
Dichlorine monoxide has the molecular formula Cl2O and is an inorganic compound. It’s a reddish-yellow gas. It was first synthesized in 1834 by Antoine Jérôme Ballard, who also determined its composition with Gay-Lussac. It is sometimes referred to as chlorine monoxide in older literature, which can be confusing because that name now refers to the neutral species ClO. It’s highly reactive and unstable.
It exists at room temperature as a brownish-yellow gas that is soluble in both water and organic solvents. It is unusually stored in frozen form as hydrate. It is a chemical compound that belongs to the chlorine oxide family and is the anhydride of hypochlorous acid. It is a powerful oxidizer and chlorinator. It’s a wood bleach, a biocide, and a swimming pool treatment.
Structure
Lewis structure of Dichlorine monoxide (Cl2O) contains two Cl-O bonds. The oxygen atom is the center atom and both chlorine atoms are located around that center oxygen atom.
Preparation
The earliest method of synthesis was to treat mercury(II) oxide with chlorine gas. However, this method is expensive, as well as highly dangerous due to the risk of mercury poisoning.
2 Cl2 + HgO → HgCl2 + Cl2O
A safer and more convenient method of production is the reaction of chlorine gas with hydrated sodium carbonate at 20–30°C.
2 Cl2 + 2 Na2CO3 + H2O → Cl2O + 2 NaHCO3 + 2 NaCl
2 Cl2 + 2 NaHCO3 → Cl2O + 2 CO2 + 2 NaCl + H2O
This reaction can be carried out in the absence of water, but it requires heating to 150–250°C; because dichlorine monoxide is unstable at these temperatures, it must be continuously removed to avoid thermal decomposition.
2 Cl2 + Na2CO3 → Cl2O + CO2 + 2 NaCl
Preparing dichlorine monoxide with mercury is an older method that is not preferred for commercial purposes because it is expensive and poses a higher risk of mercury poisoning.
Reactions
Dichlorine monoxide is very soluble in water and forms an equilibrium with HOCl. Although the rate of hydrolysis is slow enough to allow the extraction of Cl2O with organic solvents such as CCl4, the equilibrium constant eventually favors the formation of hypochlorous acid.
2 HOCl ⇌ Cl2O + H2O K (0°C) = 3.55×10-3 dm3/mol
Despite this, it has been proposed that dichlorine monoxide is the active species in HOCl reactions with olefins and aromatic compounds, as well as in drinking water chlorination.
Explosive properties
Although there has been little recent research into this behavior, dichlorine monoxide is explosive. An electric spark could not detonate room temperature oxygen mixtures until they contained at least 23.5 percent Cl2O, which is an extremely high minimum explosive limit. There have been conflicting reports of it exploding when exposed to bright light. Heating temperatures above 120°C, as well as rapid heating at lower temperatures, appear to cause explosions. Liquid dichlorine monoxide has been reported to be shock-sensitive.