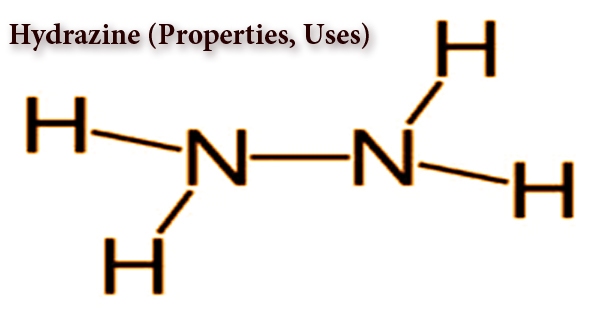
Hydrazine is a colorless, flammable, highly poisonous and unstable substance that quickly mixes with water molecules in the air to form hydrazine hydrate. The chemical formula N2H4 is an inorganic compound; it is also very significant for industrial applications, such as rocket fuel, photographic growth, and fuel cells. In the synthesis of various pesticides, hydrazine is used as a basis for blowing agents that create holes in foam rubber and as an inhibitor of corrosion in boilers. It is a colorless liquid with an odor similar to ammonia. It has a 2.0°C (35.6°F) melting point and a 113.5°C (236.3°F) boiling point. In order to form the hydrate N2H4·H2O, it readily absorbs moisture. In the preparation of polymer foams, hydrazine is primarily used as a foaming agent, but its uses also include its use as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for propulsion in spacecraft.
In 1887, hydrazine was first isolated from organic compounds; it has a 99°F flash point value. In the event that traces of air are present during the distillation process, it explodes. It is poisonous to tissues and is corrosive. By inhibiting monoamine oxidase (MAO), an enzyme that catalyzes the deamination and inactivation of some calming neurotransmitters such as norepinephrine and dopamine, hydrazine and its derivatives exhibit antidepressant properties in biological applications. In psychiatry, because of the advent of tricyclic antidepressants, the use of hydrazine derivatives is limited.
Raschig first demonstrated the preparation of hydrazine by the oxidation of NH3 with hypochlorite, a procedure that became the chief commercial production method. Hydrazines also refer to a class of organic compounds obtained by substituting an organic group for one or more hydrogen atoms in hydrazine. N2H4 is its molecular formula, and 32.04 g/mol is its molar mass. Some microorganisms such as yeast, bacteria and fungi manufacture hydrazine naturally, as it is an intermediate in anaerobic ammonia oxidation. Hydrazine hydrate solution may be subjected to fractionation to distill from water before azeotropic water and hydrazine become (68 percent hydrazine). The solution is subject to secondary fractionation; to alter its boiling point, add aniline and distill the aniline and water away from the water. Recovered aniline can be used for recycling; to obtain anhydrous hydrazine, the secondary fractionation solution is further subjected to three-fold fractionation.
In the ketazine process or the Pechiney-Ugine-Kuhlmann process, diamine can be obtained from hydrogen peroxide and ammonia. The reaction is as follows:
2NH3 + H2O2 → H2NNH2 + 2H2O
In order to manufacture nitrogen-nitrogen single bond and hydrogen chloride as ingredients, chloramine reacts with ammonia. The reaction is as follows:
NH2Cl + NH3 → H2NNH2 +HCl
Hydrazine is a liquid that is colorless and thick with a heavy ammonia odor. It has a 1.02 g/mL density and a 114°C boiling point; it is highly flammable and water-soluble. Hydrazine, an aqueous solution, appears as a colorless aqueous solution containing a maximum mass of 37 percent hydrazine and no more than 37 percent hydrazine. It burns with a violet-colored flame when ignited in the air. The compound is soluble in all H2O proportions and soluble in the form of alcohol. In the presence of metal catalysts such as platinum or Raney nickel, aqueous hydrazine decomposition occurs.
In 1887, hydrazine was first isolated by Curtius as a sulfate salt. Earlier, in 1875, the organic derivatives of hydrazine were prepared and described by Fischer. In 1906, Raschig prepared hydrazine by ammonia oxidation by hypochlorite. Hydrazine is actually a modest base, although its aqueous solutions are strongly alkaline. Oxidants, acids, metals and metal oxides react violently, creating a possible fire and explosion hazard. It releases poisonous gases of nitrogen oxide, ammonia, and hydrogen when heated for decomposition, which can also lead to fires and explosions.

It was used as fuel for rocket-powered fighter planes during World War II. However, hydrazine and its derivatives currently have the most important applications: as blowing agents; for insect control; in pharmaceuticals; in water treatment; and in fuel cells. Hydrazine is often used as a precursor to blowing agents. Azodicarbonamide and azobisisobutyronitrile, containing 100–200 mL of gas per gram of precursor, are unique compounds. Sodium azide, the gas-forming agent in airbags, is derived from hydrazine by reaction with sodium nitrite in a related application.
In combination with or in combination with other agricultural chemicals, such as insecticides, miticides, nematicides, fungicides, antiviral agents, attractants, herbicides or plant growth regulators, hydrazine compounds may be useful as active ingredients. It is also used as a reducing agent and in the electrolytic plating of metals on glass. Many hydrazine derivatives are used in azo dyes; in color photography as binding agents; and in explosives and ammunition primers. Hydrazine is passed over a catalyst such as iridium metal in all hydrazine mono-propellant engines, assisted by high-surface alumina (aluminium oxide), which allows it to decompose into ammonia, nitrogen gas and hydrogen gas according to the following reactions:
- N2H4 → N2 + 2H2
- 3N2H4 → 4 NH3 + N2
- 4NH3 + N2H4 → 3N2 + 8H2
Despite its medicinal uses, hydrazine is a highly toxic compound. Dermal, ocular, inhalation and ingestion provide possible routes of hydrazine exposure. Skin irritation/contact dermatitis and burning, eye/nose/throat irritation, nausea/vomiting, shortness of breath, pulmonary edema, headache, dizziness, depression of the central nervous system, lethargy, temporary blindness, convulsions and coma can be caused by hydrazine exposure. Hydrazine solutions can cause skin burns and are corrosive. When brought into contact with porous substances such as rusty surfaces, earth, wood, or fabric, it can undergo autoxidation and ignite spontaneously. Water spray, carbon dioxide, or dry chemical extinguishers can extinguish the flames.
Information Sources: