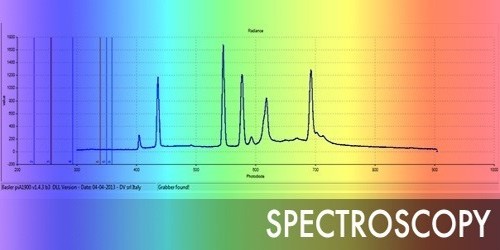
Spectroscopy is the study of light as a function of the length of the wave that has been emitted, reflected or shone through a solid, liquid, or gas. It is the technique of splitting light into its constituent wavelengths, in much the same way as a prism splits light into a rainbow of colors. To be analyzed the chemical is heated because it makes a special color of flame all chemicals make a different color spectrum than other chemicals. This can be used to see what chemicals are in a substance. Spectroscopy allows scientists to investigate and explore things that are too small to be seen through a microscope, such as molecules, and the even smaller subatomic particles like protons, neutrons, and electrons. By performing this dissection and analysis of an object’s light, astronomers can infer the physical properties of that object (such as temperature, mass, luminosity, and composition). There are special instruments to measure and analyze these light waves. Consequently, spectra are not smooth but punctuated by ‘lines’ of absorption or emission. It is concerned with the absorption, emission, or scattering of electromagnetic radiation by atoms or molecules.
Methods
Spectroscopic techniques have been applied in virtually all technical fields of science and technology. Infrared spectroscopy measures light in the infrared electromagnetic spectrum. The highlight of IR spectroscopy is that it is very useful in identifying functional groups of organic molecules. The absorption of infrared light by organic molecules causes molecular vibrations. It pertains to the dispersion of an object’s light into its component colors. The vibrational frequencies are unique to the individual functional groups. IR spectra are given graphically by transmittance(%) vs. wavenumber (cm-1)
X-ray crystallography can look at the structure of a crystalline molecule. The electron cloud of each atom diffracts the X-rays thus revealing the positions of the atoms. Various inorganic and organic molecules can be crystallized and used in this method including DNA, proteins, salts, and metals. The sample used for analysis is not destroyed.