Electrofuels are a type of renewable fuel produced by converting electrical energy into chemical energy. The process involves using electricity to power microorganisms, which can then convert carbon dioxide and water into a range of fuels, such as methane, hydrogen, or ethanol.
It is also known as e-fuels, are a type of drop-in replacement fuel and a class of synthetic fuel. They are created by combining captured carbon dioxide or carbon monoxide with hydrogen derived from renewable energy sources such as wind, solar, and nuclear power.
The process uses carbon dioxide in its manufacturing and emits roughly the same amount of carbon dioxide into the atmosphere when the fuel is burned, resulting in a low overall carbon footprint. Thus, electrofuels are a viable option for reducing greenhouse gas emissions from transportation, particularly long-distance freight, maritime, and air transport. Butanol and biodiesel are the primary targets, but other alcohols and carbon-containing gases such as methane and butane are also included.
The idea behind electrofuels is to create a carbon-neutral fuel that can replace fossil fuels in transportation and other applications. By using renewable electricity to power the microbial electrolysis process, electrofuels can potentially provide a sustainable alternative to conventional fuels that produce greenhouse gas emissions and contribute to climate change.
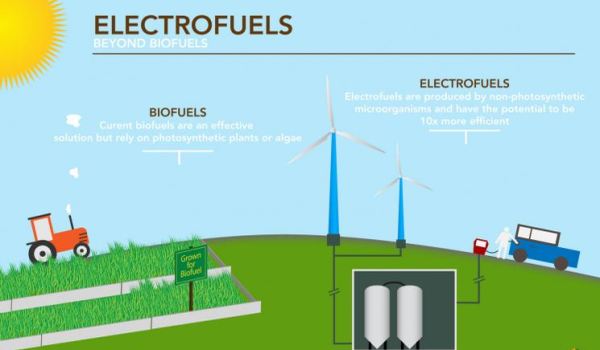
Electrofuels are seen as a promising solution to the challenge of reducing carbon emissions from transportation and industry, as they can be produced using renewable electricity from sources such as wind or solar power. They also have the potential to be more efficient than traditional biofuels, as they can be produced using microbes that can be engineered to convert carbon dioxide and water into fuels with high selectivity and yield.
However, there are still many technical and economic challenges that need to be overcome before electrofuels can become a widely adopted technology. These challenges include improving the efficiency of the electrochemical processes involved, reducing the cost of production, and finding ways to integrate electrofuel production into existing energy and transportation systems.
Electrofuels are still in the early stages of development and there are many challenges that need to be overcome, such as improving the efficiency of the microbial electrolysis process and reducing the cost of production. However, they have the potential to play an important role in reducing our reliance on fossil fuels and mitigating climate change.