Flat fullerene fragments, also known as polycyclic aromatic hydrocarbons (PAHs), can be attractive to electrons due to their conjugated pi-electron systems. PAHs are made up of fused aromatic rings that form a delocalized electron system along the carbon backbone.
The delocalized pi-electron system in PAHs enables the formation of molecular orbitals that span the entire molecule. These molecular orbitals form a continuous energy band, giving rise to unique electronic properties. PAHs can have low-lying unoccupied molecular orbitals (LUMOs) that are relatively accessible to incoming electrons.
Researchers at Kyoto University in Japan have gained new insights into the unique chemical properties of fullerenes, which are spherical molecules made entirely of carbon atoms. They accomplished this by forming flat fragments of molecules, which surprisingly retained and even enhanced some key chemical properties. The team’s findings were published in the journal Nature Communications.
“Our work could lead to new opportunities in a wide range of applications, such as semiconductors, photoelectric conversion devices, batteries, and catalysts,” says Aiko Fukazawa, group leader at the Institute for Integrated Cell-Material Sciences (iCeMS).
Our work could lead to new opportunities in a wide range of applications, such as semiconductors, photoelectric conversion devices, batteries, and catalysts.
Aiko Fukazawa
Buckyfullerene (or simply ‘buckyball’) is a molecule composed of 60 carbon atoms bonded together to form a spherical shape. It was named after structural similarities to the geodesic domes designed by renowned architect Buckminster Fuller, and its unique structure has piqued the interest of scientists for decades. Buckminsterfullerene and related spherical carbon clusters with varying numbers of carbon atoms are referred to colloquially as fullerenes, after Fuller’s surname.
One of their most intriguing properties is their ability to accept electrons, which is known as reduction. Fullerenes and their derivatives have been extensively studied as electron-transporting materials in organic thin-film transistors and organic photovoltaics due to their electron-accepting nature. Nonetheless, fullerenes are a unique class of materials when compared to other conventional organic electron acceptors due to their ability to accept multiple electrons.
Theoretical chemists have proposed three possible factors that might be behind fullerene’s electron-accepting ability: the high symmetry of the entire molecule, its carbon atoms with pyramidally arranged bonds, and the presence of pentagonal substructures distributed among six-membered rings.
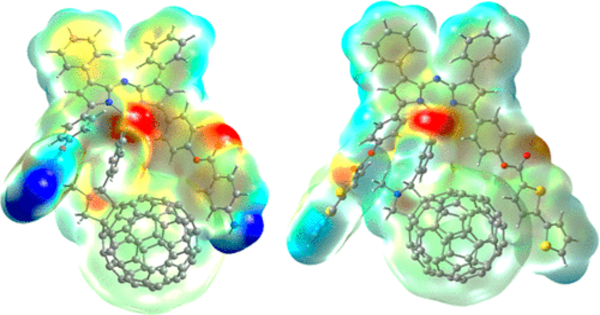
The Kyoto team concentrated on the impact of the pentagonal rings. They designed and synthesized flattened fragments of fullerene and demonstrated experimentally that these molecules could accept up to the same number of electrons as the number of five-membered rings in their structure without decomposition.
“This unexpected discovery emphasizes the critical importance of the pentagonal substructure in generating stable multi-electron accepting systems,” Fukazawa says.
Experiments also revealed that the fragments absorb more ultraviolet, visible, and near-infrared light than fullerene, which has a more limited absorbance. This could pave the way for new applications in photochemistry, such as using light to initiate chemical reactions or developing light sensors or solar-powered systems.
The researchers will now investigate the applications of their flat fullerene fragments in a wide range of electron-transfer processes. It is unusual to obtain such high electron-accepting ability in carbon-only molecules, avoiding the usual requirement to introduce other electron-withdrawing atoms or functional groups onto a carbon-based framework. Exploring the effects of incorporating other atoms or chemical groups, on the other hand, may yield more control and versatility in chemical properties.
“We hope to pioneer the science and technology of what we call super-electron-accepting hydrocarbons by exploiting their high degree of freedom to investigate the effects of structural modifications,” Fukazawa says.