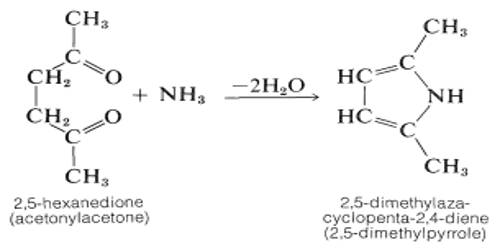
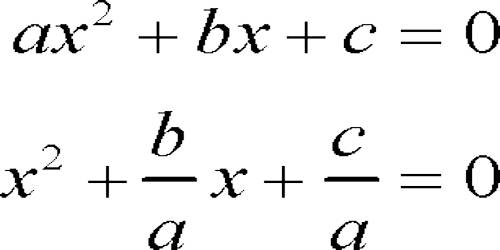A dicarbonyl is a molecule containing two carbonyls (C=O) groups. It is any metal carbonyl-containing two carbonyl groups per molecule. Although this term could refer to any organic compound containing two carboxyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Most of the 1,2-dicarbonyl compounds are yellow.
α-Dicarbonyl compounds condense with thioglycolic acid esters in the presence of sodium alkoxide to give thiophene-2,5-dicarboxylic acid derivatives. α-Dicarbonyl compounds (α-DCs), which act as precursors for advanced glycation end products in foods and in vivo, are toxic compounds formed from carbohydrates via caramelization and the Maillard reactions during thermal processing of foods. The highest concentration of total α-DCs was found in the fructose-threonine model system and the lowest concentration in the lactose-cysteine model system. The Maillard reaction is the most basic chemical reaction occurring in many foods, in the form of a non-enzymatic browning reaction between reducing sugar and amino acid.
Ethanedial is unusual in being yellow in the liquid state, but green in the vapor state. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. Various α-dicarbonyl compounds condense with thioglycolic acid esters in the presence of sodium alkoxide to give thiophene-2,5-dicarboxylic acid derivatives. The acidity of β-dicarbonyl compounds is considerably higher than that of monocarbonyl compounds and other dicarbonyl compounds. An analogous reaction occurs with diphenylethanedione, which results in the carbon-skeleton rearrangement. This is one of the few carbon-skeleton rearrangements brought about by basic reagents, and is known as the “benzilic acid rearrangement”.
In the keto-enol equilibrium of β-dicarbonyl compounds, the (mono)enolic form usually exceeds the keto form. 2,4-Pentadione, for instance, consists of about 76 percent enolic form. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical (dialdehydes, diketones, diesters, etc.) or unsymmetrical (keto-esters, keto-acids, etc.).