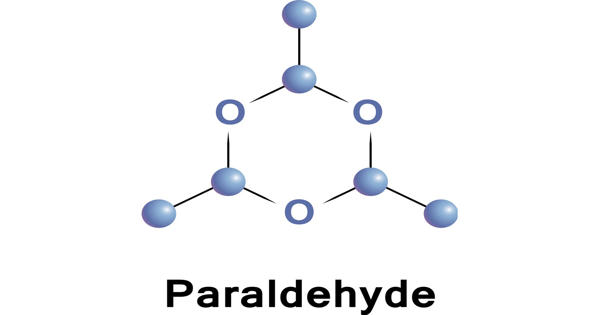
Paraldehyde is the cyclic trimer of acetaldehyde molecules. It is a liquid made by treating acetaldehyde with acid, used medicinally as a sedative, hypnotic, and anticonvulsant. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde.
Paraldehyde appears as a clear colorless liquid with a pleasant odor. It is a colourless liquid, it is sparingly soluble in water and highly soluble in ethanol. Its flash point is 96°F and melting point is 54°F. Paraldehyde slowly oxidizes in air, turning brown and producing an odour of acetic acid. It quickly reacts with most plastics and rubber.
Paraldehyde is a colorless liquid of disagreeable taste and pungent odor used in medicine as a sedative–hypnotic drug and in chemistry in the manufacture of organic chemicals.
Paraldehyde was first observed in 1835 by the German chemist Justus Liebig; its empirical formula was determined in 1838 by Liebig’s student Hermann Fehling. Paraldehyde was first synthesized in 1848 by the German chemist Valentin Hermann Weidenbusch (1821–1893), another student of Liebig; he obtained paraldehyde by treating acetaldehyde with acid (either sulfuric or nitric acid). It has uses in industry and medicine. It is produced for commerce by polymerizing acetaldehyde with a trace of sulfuric acid; the resulting liquid is then neutralized with calcium carbonate and purified by fractional distillation.

Reactions
Heated with catalytic amounts of acid, it depolymerizes back to acetaldehyde:
C6H12O3 → 3CH3CHO
Since paraldehyde has better handling characteristics, it may be used indirectly or directly as a synthetic equivalent of anhydrous acetaldehyde (b.p. 20 °C). For example, it is used as-is in the synthesis of bromal (tribromoacetaldehyde):
C6H12O3 + 9 Br2 → 3 CBr3CHO + 9 HBr
Paraldehyde is a trioxane that is 1,3,5-trioxane substituted by methyl groups at positions 2, 4 and 6. It is most useful for recalcitrant cases and is an older drug for treatment of acute alcoholic dementia. It has a role as a sedative.
Information Source: