Introduction:
Mercury poisoning (also known as mercurialism, hydrargyria, Hunter-Russell syndrome, or acrodynia when affecting children) is a disease caused by exposure to mercury or its toxic compounds. Mercury is a cumulative heavy metal poison which occurs in its elemental form, inorganically as salts, or organically as organ mercury compounds; the three groups vary in effects due to differences in their absorption and metabolism, among other factors. However, with sufficient exposure all mercury-based toxins damage the central nervous system and other organs or organ systems such as the liver or gastrointestinal tract.
Mercury poisoning is the ill effects on human’s nervous system and other bodily systems due to the over-exposure of mercury. Mercury is a neurotoxin, meaning it affects the nervous system.
Identification:
• Chemical Name: Mercury
• Regulatory Name: Mercury
• Formula: Hg
• DOT Label: Corrosive
Mercury toxicity may occur when a person is exposed to toxic amounts of mercury due to:
Breathing airborne mercury vapors
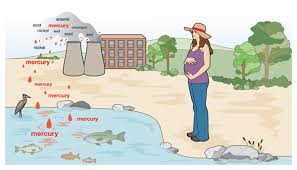
Eating food (usually fish or shellfish) contaminated with methyl mercury.
Drinking water contaminated with mercury (a relatively rare cause of poisoning)
Release of mercury from dental work and medical treatments
Main Sources of mercury poisoning:
• Drinking contaminated water
• Eating fish contaminated with mercury
• Leaching of mercury from badly fitting dental fillings.
• From vaccinations containing thimerosal.
Heavy metal toxicity from drinking contaminated water:
Arsenic has been found in tap water in levels substantially exceeding the EPA safe limit in several areas of the country.
Toxicity from eating contaminated fish: This is one of the main sources of mercury poisoning and one which is attracting a great deal of recent attention.
Mercury Toxicity from dental fillings: Dental amalgams, or “silver fillings” contains about 50% mercury along with silver, tin, copper and zinc. Mercury tends to leach from the amalgam with release of mercury vapor so over time there is a real risk of a toxic load to the body posing a real health risk.
Signs and symptoms:
The nervous system is very sensitive to the effects of mercury. Exposure to mercury can result in:
Changes in vision or hearing
Insomnia
Weakness
Memory problems
Headache
Nausea
Vomiting
Diarrhea
Increases in blood pressure or heart rate
Eye irritation
Irritability
Nervousness
Breathing problems
Painful mouth
Abdominal pain
Fever and/or chills
Erythrism (nervousness, irritability, mood instability, blushing)
Tremor
Suicidal tendency
Speech disorders
Visual disturbance
Renal damage
Adverse outcome of pregnancy
Symptoms of mercury poisoning in humans:
1. Psychological disturbances
Angry fits, short term memory loss, low self esteem, inability to sleep, loss of self-control, sleepiness. Loss of an ability to learn new things, doing things by rote.
2. Oral Cavity problems
Inflammation of the mouth, loss of bone around teeth, ulcerated gums and other areas in the mouth, loose teeth, darkening of gums, taste of metal, bleeding of gums.
3. Digestive tract problems
Cramps, inflamed colon, GI problems, Diarrhea and other digestive problems.
4. Cardiovascular problems
Weak pulse, blood pressure changes, chest pain, or feeling of pressure in the chest area.
5. Respiratory problems
Weakness and problems with breathing, Emphysema, Coughing persistently.
6 Neurological Problems
Headaches, vertigo, tinnitus, shaking in various areas of the body (eye lids, feet etc)
Causes of mercury poisoning:
Mercury poisoning is caused by sufficient exposure to elemental mercury or mercury compounds.mercury poisoning can be caused by any number of methods of exposure. Amalgam dental fillings are a main cause, other causes are eating fish that have been exposed to mercury in the environment, industrial and work place exposures such as those in the paint industry.
Some other sources of mercury are cosmetics.
Mercury toxicity may occur when a person is exposed to toxic amounts of mercury due to:
Breathing airborne mercury vapors
Eating food (usually fish or shellfish) contaminated with methyl mercury
Drinking water contaminated with mercury (a relatively rare cause of poisoning)
Release of mercury from dental work and medical treatments.
Classification of mercury poisoning:
Symptoms Characteristic of Chronic, Low-Dose Exposure
• Erythrism (nervousness, irritability, mood instability, blushing)
• Tremor
• Personality change
• Suicidal tendency
• Paraesthesia
• Impaired hearing
• Speech disorders
• Visual disturbance
• Abnormal reflexes
• Disturbed gait
• Gingivitis (inflammation of the gums)
• Impaired nerve conduction
• Renal damage
• Adverse outcome of pregnancy
• Infertility
• Pneumonitis (lung disease)
• Glioblastoma (brain cancer)
• Immune system dysfunction
Symptoms Characteristic of Acute, High-Dose Exposure
• Mouth pain
• Abdominal pain
• Vomiting
• Excessive salivation
• Anuria (urine production stops)
• Uraemia (urine products appearing in the blood)
• Nephritis (kidney disease leading to kidney failure)
• Anorexia (lack of appetite)
• Ataxia (difficulty in moving)
Physical properties of mercury:
Mercury is a transition metal. A transition metal is one of the elements found between Groups 2 (IIA) and 13 (IIIA) on the periodic table.
SYMBOL
Hg
ATOMIC NUMBER
80
ATOMIC MASS
200.59
FAMILY
Group 12 (IIB)
Transition metal
PRONUNCIATION
MER-kyuh-ree
Mercury is the only liquid metal. In fact, there is only one other liquid element, bromine. Bromine is a non-metal. Mercury can be frozen (changed into a solid) at a temperature of –38.85°C (–37.93°F). It can be changed into a gas (“boiled”) at 365.6°C (690.1°F). Its density is 13.59 grams per cubic centimeter.
Mercury has two physical properties of special interest. First, it has very high surface tension. Surface tension is a property of liquids that make them act like they are covered with a skin.
Chemical properties:
Mercury is also a very good conductor of electricity. Mercury that is released into the environment will remain there indefinitely. The form that mercury exists in (organic or inorganic) may change with time. Some or all of released organic mercury will slowlydecomposeto become inorganic mercury. Some portion of released inorganic mercury will be slowly transformed into organic mercury by bacteria in soil or water.
Mercury is not flammable and does not have an odor. Some mercury salts and organic compounds are soluble in water, depending on the chemical species. Mercury is moderately active. It does not react with oxygen in the air very readily. It reacts with some acids when they are hot, but not with most cold acids.
Mercury (chemical symbol Hg) is an amazing element. It is one of the few metals which is liquid at room temperature. It is extremely volatile, meaning it can easily be converted into gaseous form. Because of its unique properties, mercury is used in many different applications including devices such as thermometers, thermostats, fluorescent lights, and switches. It is also used in paints, preservatives, and some pesticides.
Chemical reactions of mercury:
Reaction of mercury with air
Mercury metal reacts in air at about 350°C to form mercury(II) oxide.
2Hg(s) + O2(g) → 2HgO(s) [red]
Reaction of mercury with water
Mercury does not react with water under normal conditions.
Reaction of mercury with the halogens
Mercury metal reacts with fluorine, F2, chlorine, Cl2, bromine, Br2, or iodine, I2, to form the dihalides mercury(II) fluoride, HgF2, mercury(II) chloride, HgCl2, mercury(II) bromide, HgBr2, or mercury(II) iodide, HgI2, respectively.
Hg(l) + F2(g) → HgF2(s) [white]
Hg(l) + Cl2(g) → HgCl2(s) [white]
Hg(l) + Br2(l) → HgBr2(s) [white]
Hg(l) + I2(s) → HgI2(s) [red]
Reaction of mercury with acids
Mercury does not react with non-oxidizing acids but does react with concentrated nitric acid, HNO3, or concentrated sulphuric acid, H2SO4, to form mercury(II) compounds together with nitrogen or sulphur oxides.
Mercury dissolves slowly in dilute nitric acid to form mercury(I) nitrate, mercurous nitrate, Hg2(NO3)2.
Reaction of mercury with bases
Mercury does not react with alkalis under normal conditions
Compounds:
Mercuric arsenate (HgHAsO4): waterproofing paints
Mercuric benzoate (Hg(C7G5O2)2): medicine; used to treat syphilis
Mercuric chloride, or mercury bichloride, or corrosive sublimate (HgCl2): disinfectant, tanning of leather, spray for potato seedlings (to protect from disease), insecticide, preservation of wood, embalming fluid, textile printing, and engraving
Mercuric cyanide (Hg(CN)2): germicidal soaps (soaps that kill germs), photography
Mercuric oxide (HgO): red or yellow pigment in paints, disinfectant, fungicide (to kill fungi), perfumes and cosmetics
Mercuric sulfide (HgS): red or black pigment in paints
Mercurous Mercurous chromate (Hg2CrO4): green pigment in paints
Mercurous iodide (Hg2I2): kills bacteria on the skin
Mercury in Industry
Mercury poisoning used to be widespread in such industries as mirror making and cinnabar (mercury ore) mining.
In Western countries, the use of mercury in industrial processes has almost disappeared. In modern times, exposure, and therefore toxicity is limited mainly to dentistry; thermometer, barometer and mercury arc equipment manufacture; pigment, fungicide, insecticide and dry cell battery manufacture. Mercury compounds have in the past been used as diuretics, anti-infective, laxatives, eye and skin treatments, but these uses have now been superseded by more appropriate drugs. Mercury compounds were once used extensively in the production of paper.
How the Body Gets Rid of Mercury
Disposal of the body’s burden of mercury is via the urine and faces, although minute amounts are detectable in expired air. Excretion via the liver occurs in bile and reabsorption of some of this mercury does take place. However, the kidney is equipped with an efficient, energy-dependant mechanism for disposing of metals such as mercury.
Kidney tissue contains a thiol-rich protein called metallothionein; exposure to toxic metals triggers the production of this protein which binds tightly to the metal, retaining it in the kidney tissue in a relatively harmless form. As long as the kidney’s capacity for production of metallothionein is not overwhelmed, mercury excretion can eventually balance intake, thereby limiting worsening of symptoms. However, acute high doses of mercury, or an increase in the chronic dose level can readily precipitate renal failure, one of the classic symptoms of mercury poisoning.
Elemental mercury:
Elemental mercury can be found in
• Electrical switches
• Fluorescent light bulbs
• Older dental fillings
• Some medical equipment.
Elemental mercury is usually quite harmless if touched or swallowed. It is so thick and slippery that it usually falls off your skin or out of your stomach without being absorbed.
Breathing in elemental mercury will cause symptoms right away (acute) if enough mercury is breathed in. Symptoms will also occur over time (chronic) if little amounts are inhaled every day. If this occurs, symptoms may include:
• Metallic taste
• Vomiting
• Difficulty breathing
• Bad cough
• Swollen, bleeding gums
INORGANIC MERCURY:
Unlike elemental mercury, inorganic mercury is usually poisonous when swallowed. Depending on the how much is swallowed, symptoms may include
• Burning in the stomach and throat
• Bloody diarrhea and vomiting
If inorganic mercury enters your blood stream, it can attack the kidneys and brain. Permanent kidney damage and failure may occur. A large overdose may cause massive blood and fluid loss from diarrhea, kidney failure, and death.
Inorganic mercury can found in:
• Chemistry labs
• Some disinfectants
• Folk culture medicines
Organic mercury:
Organic mercury can cause sickness if breathed in, eaten, or placed on the skin for long periods of time. Usually organic mercury causes problems over years or decades, not immediately. In other words, being exposed to small amounts of organic mercury every day for years will likely cause symptoms to appear later. Regardless, a single large exposure can also cause problems.
Long-term exposure will likely cause neurological symptoms, including:
• Numbness or pain in certain parts of your skin
• Uncontrollable shake or tremor
• Inability to walk well
• Blindness and double vision
• Memory problems
• Seizures and death (with large exposures)
Organic mercury can be found in:
• Thimerosal
• Fumes from burning coal converted into organic mercury by certain organisms
• Fish that have eaten a form of organic mercury called methyl mercury — see article on methyl mercury
Applications:
• For the manufacturing of industrial chemicals or for electrical and electronic applications.
• In thermometers.
• As mercury sphygmomanometers, a blood pressure meter.
• Thimerosal, an organic compound is used as preservative in vaccines and tattoo inks.
• As mercury barometers, diffusion pumps, coulometer, and many more laboratory instruments.
• In mercury arc rectifier, a type of electrical rectifier that converts alternate current into direct current.
• In mercury-vapor lamps and some neon advertising signs and fluorescent lamps.
• Once used as coolant for nuclear reactors, which has been replaced by sodium and also in the amalgamation process of refining gold and silver ores.
• As folk medicine and ceremonial purposes that involves ingestion, injection, or the sprinkling of elemental mercury around the home.
• In mercury switches, mercury cells and chlorine production, electrodes, batteries, and catalysts.
• As herbicides, insecticides, dental amalgams and liquid mirror telescopes.
Acute Exposure
Many acute health effects are associated with exposure to high levels of elemental mercury vapor. Respiratory symptoms may predominate (cough, sore throat, shortness of breath). Gastrointestinal effects are frequent in the initial set of symptoms (metallic taste, nausea, vomiting, diarrhea, abdominal pain) as are CNS effects such as headache, weakness, and visual disturbances. Children do not always respond to chemicals in the same way that adults do. Different protocols for managing their care may be needed.
Renal
Acute high-dose inhalation of elemental mercury vapor has been associated with proteinuria, nephritic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.
Cardiovascular
Acute inhalation of high levels of elemental mercury vapor can cause tachycardia and hypertension. In children, tachycardia associated with the inhalation of elemental mercury vapor might be related to a non-allergenic hypersensitivity reaction to mercury.
Gastrointestinal
A metallic taste, salivation, dysphagia, abdominal cramps, diarrhea, and nausea have been reported following inhalation of large amounts of elemental mercury vapor. Oral and dermal exposures to elemental mercury are not normally associated with GI symptoms.
Dermal
Dermal reactions associated with dermal contact with liquid elemental mercury or the vapor are rare. Acrodynia (or pink disease) is associated with hypersensitivity to mercury absorbed from vapor inhalation or dermal exposure. Symptoms of acrodynia include abnormal redness of the skin, followed by peeling of skin on the hands, nose, and soles of the feet.
CNS
Acute inhalation of mercury vapor may produce CNS effects such as headache, weakness, and visual disturbances.
Carcinogenicity
The Department of Health and Human Services (DHHS), the International Agency for Research on Cancer (IARC), and the Environmental Protection Agency (EPA) have not had sufficient evidence to classify elemental mercury as a carcinogen or a no carcinogen.
Chronic inhalation of elemental mercury vapor has not been shown to have any effect on spermatozoa in men. An increased incidence of spontaneous abortion among the wives of men chronically exposed to elemental mercury has been reported.
In female workers, menstrual disorders (dysmenorrheal) have been associated with chronic exposure to high concentrations of mercury vapor. At high levels, inhaled elemental mercury is able to cross the placental barrier, but fetotoxic or significant developmental effects have not been well studied in humans. Adverse developmental effects have been observed in animals but not
Treatment: Treatment options include:
Chelation Therapy
Chelation therapy involves putting a chemical, or chelating agent, into the bloodstream. The chelating agent combines with mercury to help remove it from the body. Chelating agents may be given by pill or by injection.
For recent ingestion, the doctor may induce vomiting, pump out the stomach (gastric lavage), or give polythiol resins to bind with the mercury.
Prevention:
To help reduce your chances of getting mercury toxicity, take the following steps:
Avoid using metallic mercury for any purpose
If you must use metallic mercury, keep it safely stored in a leak-proof container in a secure space (eg, a locking closet)
Trade in old thermometers or barometers containing mercury for new ones that do not
Carefully handle and dispose of items containing mercury (eg, thermometers, fluorescent light bulbs)
Do not vacuum or heat spilled mercury
Teach children not to play with silver liquids
Properly dispose of old medications containing mercury
Keep mercury-containing medications away from children
Learn about wildlife and fish advisories in your area from your local public health or natural resources department
Limit fish intake to recommended quantities and avoid fish known to be especially contaminated by methyl mercury
If you spill a small amount of metallic mercury (eg, a broken thermometer):
Remove children from the area.