In chemistry, an ideal solution or ideal mixture is a solution in which the gas phase exhibits thermodynamic properties analogous to those of a mixture of ideal gases. It is a solution in which the interaction between molecules of the components does not differ from the interactions between the molecules of each component. The enthalpy of mixing is zero as is the volume change on mixing by definition; the closer to zero the enthalpy of mixing is the more “ideal” the behavior of the solution becomes. We can obtain ideal solutions by mixing two ideal components that are, solute and a solvent having a similar molecular size and structure.
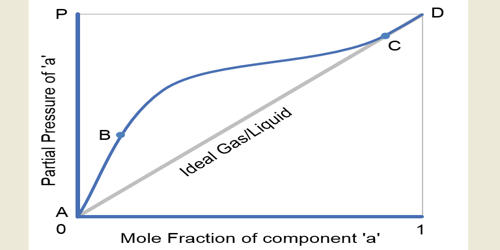
Raoult’s law states that the vapor pressure of a solvent above a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of the solvent present. The vapor pressure of the solution obeys either Raoult’s law or Henry’s law (or both), and the activity coefficient of each component (which measures the deviation from ideality) is equal to one. According to the law, ‘the mole fraction of the solute component is directly proportional to its partial pressure’. Some examples of ideal solution liquid pairs are benzene and toluene, n-heptane and n-hexane, ethyl bromide and ethyl iodide, chlorobenzene, and bromobenzene, etc.
The ideal Solutions are those which obey Raoult’s Law at all concentrations and Temperatures. The concept of an ideal solution is fundamental to chemical thermodynamics and its applications, such as the use of colligative properties.
Physical origin
An ideal solution can be obtained by mixing a solute and a solvent which consist of similar molecular structure and size. Ideality of solutions is analogous to ideality for gases, with the important difference that intermolecular interactions in liquids are strong and cannot simply be neglected as they can for ideal gases. Instead, we assume that the mean strength of the interactions is the same between all the molecules of the solution. The vapor pressures of the solutions are mathematically represented by a linear function of the molecular composition.
The properties of such solutions often are described in terms of their deviations from those of ideal solutions. More formally, for a mix of molecules of A and B, the interactions between unlike neighbors (UAB) and like neighbors UAA and UBB must be of the same average strength, i.e., 2 UAB = UAA + UBB and the longer-range interactions must be nil (or at least indistinguishable). If the molecular forces are the same between AA, AB, and BB, i.e., UAB = UAA = UBB, then the solution is automatically ideal. Solutions of acetone and carbon disulfide, on the other hand, have higher vapour pressures than those that would characterize an ideal solution.
If the molecules are almost identical chemically, e.g., 1-butanol and 2-butanol, then the solution will be almost ideal. The solute-solute interaction and solvent-solvent interaction are almost similar to the solute-solvent interaction. The concept of an ideal solution is fundamental to chemical thermodynamics and its applications, such as the use of colligative properties.