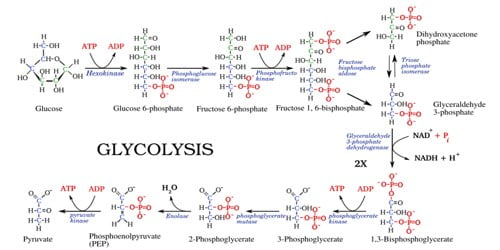
Nickel brass, (also known as German silver), is a copper alloy with nickel and often zinc. It is a gold-colored copper-zinc-nickel alloy. The usual formulation is 60% copper, 20% nickel, and 20% zinc. The color depends on the amount of nickel present. It is known as nickel silver and can be regarded as a special brass.
Nickel Brass is named due to its silvery appearance, but it contains no elemental silver unless plated. These alloys have the quality advantages of brass but are considered to be safe alloys. All modern, commercially important nickel silvers contain significant amounts of zinc and are sometimes considered a subset of brass. In most respects, nickel silvers show similar corrosion resistance characteristics to brasses, but the higher nickel versions have superior tarnish resistance and resistance to stress corrosion cracking. Nickel brass tokens are difficult to copy because the alloy is only produced for secure coinage applications.
Nickel brass alloy 2% is darker than nickel-brass alloy 6%. This is an alloy that contains copper, nickel, and zinc. The nickel gives the material an almost silver appearance. In the nineteenth century, particularly after 1868, North American Plains Indian jewelers were able to easily acquire sheets of German silver. They used them to cut, stamp, and cold hammer a wide range of accessories and also horse gear. The metal has an attractive silvery appearance rather than the typical brassy color.

Fig: Nickel-plated Brass
Uses
This material has moderate strength and fairly good corrosion resistance. This material is typically used to make musical instruments, food and beverage equipment, optical equipment, and other items where the aesthetics are an important factor.
- Nickel brass first became popular as a base metal for silver-plated cutlery and other silverware, notably the electroplated wares called EPNS (electroplated nickel silver).
- It is used in zippers, better-quality keys, costume jewelry, for making musical instruments (e.g., flutes, clarinets), and is preferred for the track in electrically powered model railway layouts, as its oxide is conductive.
- The leaded nickel brasses are used where machinability, attractive appearance, corrosion, and wear resistance are required. Common examples are cylinder lock keys, screws, gears, pinions, and other parts in clocks, cameras, and musical instruments.
- It is widely used in the production of coins (e.g. Portuguese escudo and the former GDR marks). It has a specific electromagnetic signature allowing electronic coin validators to distinguish these from other coins. Its industrial and technical uses include marine fittings and plumbing fixtures for its corrosion resistance, and heating coils for its high electrical resistance.