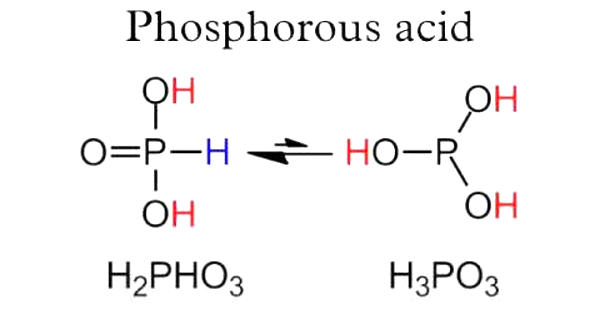
Phosphorous acid is the compound described by the formula H3PO3. It is the most important oxygen acid of phosphorus, used to make phosphate salts for fertilizers. This acid is diprotic (readily ionizes two protons), not tricrotic as might be suggested by this formula. It appears as a white or yellow crystalline solid or a solution of the solid.
Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO2H2, are called phosphonic acids.
Properties
It is a colourless or yellowish crystalline substance with a garliclike taste. Pure phosphoric acid is a crystalline solid (melting point 42.35° C, or 108.2° F); in less concentrated form it is a colourless syrupy liquid. The crude acid is prepared from phosphate rock, while acid of higher purity is made from white phosphorus.
- Density: 1.65 g/cm³
- Molecular Weight/ Molar Mass: 82 g/mol
- Boiling Point: 200 °C
- Melting Point: 73.6 °C
- Appearance: White solid, deliquescent
- Solubility: Soluble in water.
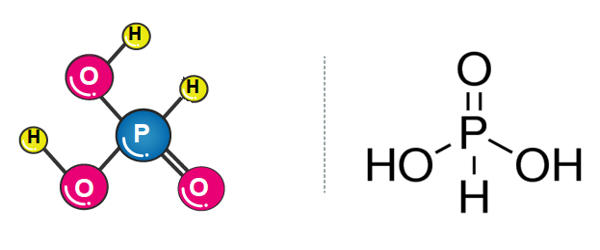
Preparation
Phosphorous acid is produced in the form of a white volatile powder by the slow combustion of phosphorus. HPO(OH)2 is the product of the hydrolysis of its acid anhydride:
P4O6 + 6 H2O → 4 HPO(OH)2
(An analogous relationship connects H3PO4 and P4O10).
On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam:
PCl3 + 3 H2O → HPO(OH)2 + 3 HCl
It is conveniently prepared by allowing phosphorous trichloride to react with water. In industrial synthesis PCl3 is sprayed into steam at 1900C the heat of reaction is used to distill off the hydrogen chloride and excess water vapour.
Uses
- The most important use of phosphorous acid (phosphonic acid) is the production of basic lead phosphite, which is a stabilizer in PVC and related chlorinated polymers.
- It is also used in dental cements, in the preparation of albumin derivatives, and in the sugar and textile industries.
- It is also used as a strong reducing agent and in the production of phosphorous acid, synthetic fibres, organophosphorus pesticides, and the highly efficient water treatment agent ATMP.
- Ferrous materials, including steel, may be somewhat protected by promoting oxidation (“rust”) and then converting the oxidation to a metalophosphate by using phosphoric acid and further protected by surface coating.
- It serves as an acidic, fruitlike flavouring in food products.
- It is used in the production of high efficient water treatment agent amino trimethylene phosphonic acid.
Information Source: