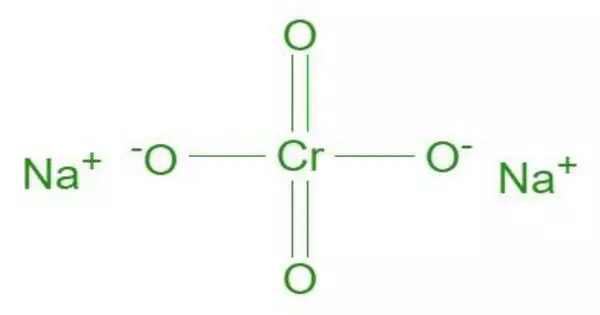
The inorganic compound sodium chromate has the formula Na2CrO4. It is an inorganic sodium salt with a 2:1 ratio of sodium and chromate ions. It exists as a yellow hygroscopic solid that can be converted into tetra-, hexa-, and decahydrates. It is used as a step in the extraction of chromium from its ores.
This substance is used as a corrosion inhibitor and in wood preservatives, drilling muds, cutting oils, water treatment, textile dyeing processes, inks, paints, and leather tanning. It functions as a carcinogen, an oxidizer, a poison, and a diagnostic agent.
Properties
Sodium Chromate is a yellowish, crystalline, inorganic compound that emits toxic chromium fumes upon heating. It is highly corrosive and is a strong oxidizing agent. Molecular weight of Sodium Chromate is 161.972 g/mol. Its density is 2.698 g/cm3. Its melting point is 792οC.
- Chemical formula: Na2CrO4
- Molar mass: 161.97 g/mol
- Appearance: yellow crystals
- Odor: odorless
- Density: 2.698 g/cm3
- Melting point: 792 °C (1,458 °F; 1,065 K) (anhydrous); 20 °C (decahydrate)
- Solubility in water: 31.8 g/100 mL (0 °C); 126.7 g/100 mL (100 °C)
- Solubility: slightly soluble in ethanol
- Solubility in methanol: 0.344 g/100 mL (25 °C)
- Crystal structure: orthorhombic (hexagonal above 413 °C)

Production and reactivity
It is obtained on a vast scale by roasting chromium ores in air in the presence of sodium carbonate:
2Cr2O3 + 4 Na2CO3 + 3 O2 → 4 Na2CrO4 + 4 CO2
This process converts chromium into a water-extractable form while leaving iron oxides behind. Calcium carbonate is typically added to the mixture to improve oxygen access and to keep silicon and aluminum impurities insoluble. Typically, the process temperature is around 1100 °C.
A mixture of chromite ore, sodium hydroxide, and sodium nitrate reacting at lower temperatures may be used for lab and small scale preparations (even 350 C in the corresponding potassium chromate system). Following its formation, the chromate salt is converted to sodium dichromate, which is the precursor to the majority of chromium compounds and materials. The industrial route to chromium(III) oxide involves sulfur reduction of sodium chromate.
Uses
In the petroleum industry, sodium chromate is used as a corrosion inhibitor. It is used in the production of chromic acid. Aside from its importance in the extraction of chromium from its ores, sodium chromate is used in the petroleum industry as a corrosion inhibitor. In the textile industry, it is also used as a dyeing auxiliary. It is a pharmaceutical used to determine the volume of red blood cells. It is a powerful oxidizing agent.
The main use of sodium chromate is to make sodium dichromate. Sodium chromate is used as an oxidant in organic chemistry, converting primary alcohols to carboxylic acids and secondary alcohols to ketones. Sodium chomrate is an extremely powerful oxidizer.
Health Hazards
Sodium chromate is primarily harmful to the respiratory tract, causing ulcers, shortness of breath, bronchitis, pneumonia, and asthma, but it can also harm the gastrointestinal tract, liver, kidneys, and immune system. When inhaled, sodium chromate can have an effect on you. It has the potential to irritate the skin. Its contact with the eyes can cause irritation. It has the potential to harm the liver and kidneys.