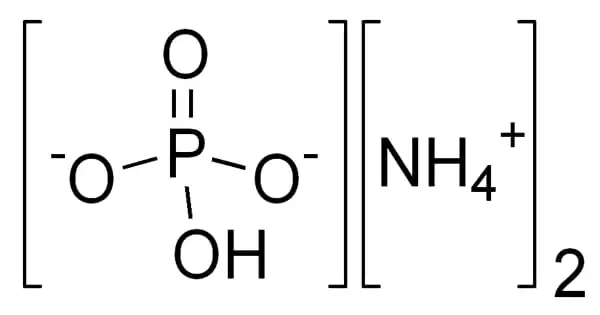
Diammonium phosphate [chemical formula (NH4)2(HPO4)] is one of several water-soluble ammonium phosphate salts that can be formed when ammonia interacts with phosphoric acid.
Diammonium phosphate (DAP) is the most extensively used phosphorus fertilizer in the world. It’s manufactured from two typical fertilizer elements, and its relatively high nutrient content and outstanding physical qualities make it a popular choice in farming and other industries.
The dissociation pressure of ammonia in solid diammonium phosphate is given by the following expression and equation:
(NH4)2HPO4(s) ⇌ NH3(g) + (NH4)H2PO4(s)
At 100 °C, the dissociation pressure of diammonium phosphate is approximately 5 mmHg.
DAP fertilizer is a great source of phosphorus (P) and nitrogen (N) for plant nutrition. Because it is extremely soluble, it dissolves fast in soil, releasing plant-available phosphate and ammonium. The alkaline pH that develops surrounding the dissolving granule is a remarkable feature of DAP.
According to CF Industries, Inc.’s diammonium phosphate MSDS, breakdown begins as low as 70 °C: “Decomposition Products That Are Dangerous: When exposed to room temperature air, it gradually loses ammonia. At roughly 70 °C (158 °F), it decomposes to ammonia and monoammonium phosphate. DAP emits phosphorus oxides, nitrogen oxides, and ammonia at 155 °C (311 °F).”

Properties
Diammonium phosphate is a fertilizer with two nutrients. It is composed of 18% nitrogen (N) and 46% phosphorus (P) as P2O5. Because its nitrogen concentration is in the form of ammonium (NH4), it is very helpful in the early stages of plant development. When used as a base fertilizer, its nitrogen content may not be sufficient to meet the needs of plants.
- Molar mass: 132.06 g/mol
- Appearance: colorless monoclinic crystals
- Density: 1.619 g/cm3
- Melting point: 155 °C (311 °F; 428 K) decomposes
- Solubility in water: 57.5 g/100 mL (10 °C) and 106.7 g/100 mL (70 °C)
- Solubility: insoluble in alcohol, acetone and liquid ammonia
Uses
DAP is a type of fertilizer. When used as plant food, it temporarily raises the pH of the soil, but over time, the treated soil becomes more acidic due to ammonium nitrification. Because its ammonium ion is more prone to change to ammonia in a high-pH environment, it is incompatible with alkaline substances. The pH of the fluid is 7.5–8 on average. The most common formulation is 18-46-0. (18 percent N, 46 percent P2O5, 0 percent K2O).
DAP has the potential to be employed as a fire retardant. It lowers the material’s combustion temperature, reduces maximum weight loss rates, and increases the creation of residue or char. Lowering the pyrolysis temperature and raising the amount of char created limits the amount of available fuel and can lead to the construction of a firebreak, which is important in controlling wildfires. It is the main element in certain popular commercial firefighting products and is used to make “fire retardant” cigarettes.
DAP is also used as a yeast nutrient in winemaking and mead-making; as a nicotine enhancer in some brands of cigarettes; to prevent afterglow in matches, in sugar purification; as a flux for soldering tin, copper, zinc, and brass; and to control the precipitation of alkali-soluble and acid-insoluble colloidal dyes on wool.
DAP is also a fire retardant. A mixture of DAP and other materials, for example, can be dispersed ahead of a fire to keep a forest from burning. After the risk of fire has passed, it becomes a food source. DAP is also utilized in a variety of industrial operations, such as metal finishing. And, it’s commonly added to wine to sustain yeast fermentation and to milk to produce cheese cultures.
Natural occurrence
The chemical occurs naturally as the extremely rare mineral phosphammite. The mineral biphosphammite has a similar dihydrogen compound. Both are associated with guano deposits.