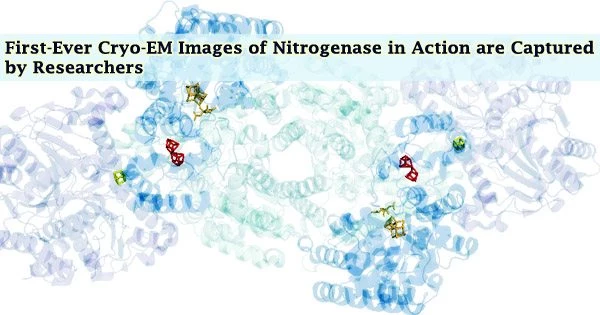
The only enzyme capable of reducing nitrogen into ammonia is nitrogenase, and it has never been possible to take high-resolution pictures of it in action. Cryogenic electron microscopy (CryoEM) was used by researchers at the University of California, San Diego to capture near-atomic-resolution images of nitrogenase undergoing catalysis. The results were published in the journal Science.
This work was accomplished through a close partnership between the groups of Professor Akif Tezcan and Assistant Professor Mark Herzik, both in UC San Diego’s Department of Chemistry and Biochemistry. While Herzik offered the cryoEM knowledge required to complete the project, Tezcan has long studied nitrogenase.
“This is a very important advance in terms of biological nitrogen fixation as well as structural biology, in general,” stated Tezcan. “To be able to obtain atomic-level-resolution pictures of an enzyme as dynamic and complex as nitrogenase in action is extremely exciting. It opens the doors to fully understanding the mechanism of this enigmatic enzyme, which has preoccupied researchers for decades.”
Understanding the enormous worldwide relevance of nitrogen fixation is necessary to comprehend the significance of these cryoEM images. For the manufacture of elements essential to life, such as proteins and DNA, all organisms need “fixed” sources of nitrogen. However, because most living things lack the nitrogenase enzyme, they are unable to convert atmospheric nitrogen into a form that can be bioprocessed.
Prior to the invention of the commercial Haber-Bosch process, which turns atmospheric nitrogen into ammonia more than a century ago, nitrogenase was practically the primary source of fixed nitrogen in the biosphere.
Ammonia manufactured industrially is mostly utilized as a fertilizer, and its invention changed farming methods in the early part of the 20th century. The Haber-Bosch process, which “turned air into bread,” has frequently been credited with being the primary cause of the world’s population explosion over the past century.
This is a very important advance in terms of biological nitrogen fixation as well as structural biology, in general. To be able to obtain atomic-level-resolution pictures of an enzyme as dynamic and complex as nitrogenase in action is extremely exciting. It opens the doors to fully understanding the mechanism of this enigmatic enzyme, which has preoccupied researchers for decades.
Professor Akif Tezcan
However, the Haber-Bosch process consumes a lot of energy because it calls for hydrogen gas at high pressures and temperatures above 400 °C. The Haber-Bosch process uses 1% to 2% of all energy produced globally.
Additionally, the procedure poses environmental issues, such as nitrate leaking into groundwater and increased nitrous oxide emissions. The comparison between nitrogenase to the Haber-Bosch process is a fundamental issue in biological nitrogen-fixation research.
How does the enzyme catalyze nitrogen reduction at ambient temperatures and pressure whereas the industrial process requires such extreme conditions?
“If we can understand the mechanism of nitrogenase, we may not only figure out why nature evolved it to be such a complex enzyme, but we might also uncover design principles for ammonia production in a more cost-effective and environmentally friendly fashion,” stated Tezcan.
Despite the fact that much is known about the structure of nitrogenase, until recently no one has been able to take atomic-resolution pictures of the enzyme when it is “turning over” or catalyzing the conversion of atmospheric nitrogen into ammonia, partly because of technological restrictions.
Although X-ray crystallography can be used to produce atomic-resolution photographs of proteins, this technique necessitates that the proteins be fixed in place within a crystal, making it impossible to record nitrogenase in action.
In order to convert nitrogen into a single ammonia molecule using nitrogenase catalysis, many enzyme components must interact with one another and then repeatedly dissociate from one another. There is no such thing as a stagnant process.
Thanks to recent developments in hardware and data processing, cryoEM not only enables researchers to record protein structures without needing to fix them in crystals, but also to do so with atomic resolution. To see the minute enzymatic catalysis-related changes, such high resolution is required.
These developments prompted Tezcan and graduate student Hannah Rutledge to think about studying nitrogenase’s catalytic function using cryoEM. And for this, they enlisted the aid of local cryoEM expert Mark Herzik, along with Brian Cook and Hoang Nguyen from his team.
“This was both an exciting and technologically challenging project to pursue, during the pandemic no less. Although cryoEM is a very capable technique, few studies have reported on enzymes as they undergo catalysis. The critical insights and technological developments in this study not only pave the way for future explorations of the nitrogenase mechanism but enzymes in general,” stated Herzik.
Rutledge and Herzik collaborated closely to prepare a large number of cryoEM samples. The samples were produced in an anaerobic glove box since nitrogenase is oxygen-sensitive, then swiftly transferred and instantly frozen to stop any deterioration.
The researchers eventually gathered more than 15,000 videos, totaling more than 20 million distinct molecules at various phases of catalysis. The teams had to go through several terabytes of data over the course of roughly a year, first eliminating low-quality photos before identifying and categorizing every particle.
Eventually, they were able to capture the first photos of nitrogenase mid-turnover at atom resolution. Several unexpected characteristics of nitrogenase that had not before been seen in X-ray structures were disclosed by the cryoEM structures.
The new findings offer fresh mechanistic theories for nitrogenase catalysis, which is significant. Tezcan and Herzik intend to work together for several years to test these theories and fully comprehend nitrogenase’s catalytic mechanism.
“This is only the beginning,” stated Tezcan. “We have a picture of the whole enzyme now, not just one specific part, during catalytic action. This will really open the floodgates to further research in understanding how nitrogenase works and, potentially, down the road, developing more efficient processes for producing fixed nitrogen.”
Funding provided by National Institutes of Health grants R01-GM099813, R35-GM138206 and T32-GM008326; NASA grant 80NSSC18M0093; and the Searle Scholars Program. CryoEM experiments were conducted at UC San Diego’s CryoEM facilities as well as the Stanford-SLAC Cryo-EM Center.