Barium nitrate is a smooth, white crystalline solid; it is the chemically formulated inorganic compound Ba(NO3)2. Non-combustible however accelerates the combustion of fuel materials. If large quantities are involved in a fire or fine division of the combustible material, an explosion can result. It is colorless, poisonous, and water-soluble like other barium salts. It burns and is an oxidizer with a green flame; the compound is widely used in pyrotechnics.
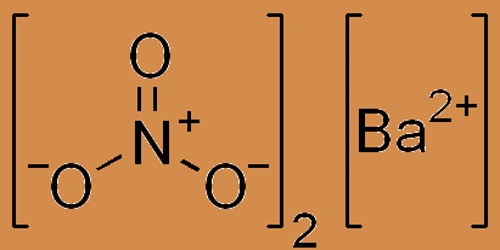
Barium nitrate forms white crystals, which are water-soluble at 20 ° C. It is formed by the addition of nitric acid to barium carbonate or barium hydroxide. It occurs as the very rare mineral nitrobarite, naturally. Barium nitrate decomposes to a barium oxide at high temperatures:
2Ba(NO3)2 → 2BaO + 4NO2 + O2
The barium nitrate is crystallized at low temperatures, after carefully extracting any embedded sulfur and the heavy metals. It is toxic, is an irritant to the respiratory system, and is harmful when combined with flammable materials. Barium oxide plus zinc, aluminum, and magnesium alloys are toxic and are combustible (paper, oil, and wood), acid, and oxidizers. Barium nitrate mixed with aluminum powder, a formula for flash powder, is extremely explosive. It mixed with thermite to make Thermate-TH3, employed in military thermite grenades. Barium nitrate mixed with aluminum powder, a formula for flash powder, is extremely explosive. It’s mixed with thermite to make Thermate-TH3, utilized in military thermite grenades.
Yet barium nitrate in the presence of glass is noncorrosive. It is also used in the barium oxide production process, in the vacuum tube industry, and in pyrotechnics for green fire. The Eastern European patent literature explains the preparation of Ba(NO3)2 by double conversion of barium chloride and sodium nitrate, or calcium nitrate. Magnesium plus barium oxide plus zinc, aluminum and magnesium alloys, combustibles (paper, oil, wood), acids, and oxidizers are dangerous. Mixtures of neatly separated aluminum-magnesium alloys are readily ignitable and highly friction or impact sensitive.
Sulfate salt solutions such as Epsom salts or sodium sulfate can be provided as first aid for barium poisoning, as they precipitate the barium as the insoluble (and non-toxic) barium sulfate. Baratol, a combination of barium nitrate and TNT, is used in wave forming devices such as plane-wave lenses with the low detonation velocity explosive. The nitric acid/barium metal reaction is one way and the BaO or BaCO3 reaction is another. Barium nitrate should be contained in a container that is securely closed, in a cool, dry, ventilated environment safe from physical harm. It should be isolated from the sun, ignition sources, incompatible liquids, combustibles, and readily oxidizable organic or other materials. Not to store barium nitrate on wood l oors or with food and beverages.
Although contact with the skin or eyes is less harmful than ingestion or inhalation, discomfort, scratching, redness and pain may still result. With just a little of the nitrite, it decomposes at higher temperatures to give more oxide and peroxide. Prolonged periods of barium nitrate exposure are known to cause liver damage (anemia and likely methemoglobinemia), spleen, kidney, bone marrow, and CNS. Staff should cause vomiting immediately as directed by medical personnel following accidental exposures to barium nitrate by swallowing, swallow, or inhalation. Never give an unconscious person food by mouth; get medical attention right away.
Information Sources: