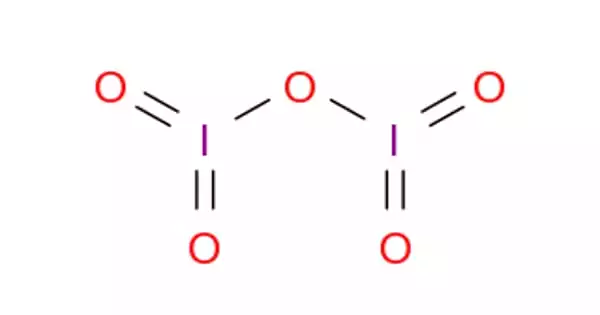
Iodine pentoxide is the chemical compound with the formula I2O5. It is an iodine oxide. This iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is the anhydride of iodic acid. It can be used in gasometric analysis to measure carbon monoxide. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:
2HIO3 → I2O5 + H2O
Properties
It is white crystals; hygroscopic; density 4.98 g/cm3; decomposes around 300°C; highly soluble in water; soluble in nitric acid; insoluble in ethanol, ether and carbon disulfide.
- Melting point: 300-350°C (dec.)
- Density: 5.08 g/mL at 25 °C
- Storage temp.: Store at +5°C to +30°C.
- Form: Crystalline
- Color: White to cream
- Specific Gravity: 5.08
- Water Solubility: soluble
Structure
I2O5 is bent with an I–O–I angle of 139.2°, but the molecule has no mirror plane so its symmetry is C2 rather than C2v. The terminal I–O distances are around 1.80 Å and the bridging I–O distances are around 1.95 Å.

Preparation
Iodine pentoxide is prepared by oxidizing iodine with nitric acid. Intermediate product iodic acid is converted to iodine pentoxide by dehydrating iodic acid at 200°C. Iodine pentoxide is prepared by dehydration of iodic acid at 240°C.
2HIO3 → I2O5 + H2O
Reactions
Iodine pentoxide easily oxidizes carbon monoxide to carbon dioxide at room temperature.
5 CO + I2O5 → I2 + 5 CO2
This reaction can be used to analyze the concentration of CO in a gaseous sample.
I2O5 forms iodyl salts, [IO2+], with SO3 and S2O6F2, but iodosyl salts, [IO+], with concentrated sulfuric acid.
Iodine pentoxide decomposes to iodine (vapor) and oxygen when heated to about 350 °C.
Uses
Iodine pentoxide is used for analysis of carbon monoxide and for CO removal from air. It also is used as an oxidizing agent in other oxidation reactions.