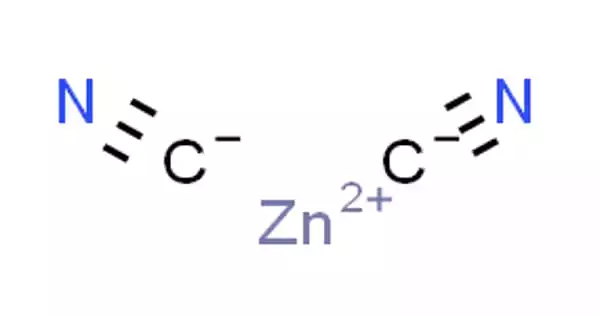
The inorganic compound zinc cyanide has the formula Zn(CN)2. It has the appearance of a white powder. It is a white solid that is primarily used for electroplating zinc but also has more specialized applications in organic compound synthesis. It’s not soluble in water. It is toxic when inhaled or ingested.
Zinc cyanide is a chemical that is used in medicine, metal plating, and chemical analysis. It is used in zinc electroplating. It is used in the Gatterman reaction to introduce formyl groups into aromatic compounds as well as to prepare 2-hydroxy-1-nafthaldehyde and mesitaldehyde. It is also used in analytical chemistry for chemical analysis. It also serves as a catalyst for the cyanosilylation of aldehydes and ketones. Furthermore, it reacts with 1,3,5-triethyl-benzene to form 2,4,6-triethyl-benzaldehyde.
Properties
- Formula: Zn(CN)2
- Formula Weight: 117.4
- 2Melting point: 800° dec.
- Density: 1.852
- Storage & Sensitivity: Hygroscopic, Store under Argon, Ambient temperatures.
- Solubility: Soluble in alkalies, potassium cyanide and ammonia. Insoluble in water and most solvents.

Structure
Zinc adopts a tetrahedral coordination environment in Zn(CN2)2, which is linked by bridging cyanide ligands. Two “interpenetrating” structures make up the structure (blue and red in the picture above). These motifs are sometimes referred to as “expanded diamondoid” structures. Some forms of SiO2 have a similar structure, with the tetrahedral Si centers connected by oxides. The cyanide group exhibits head-to-tail disorder, with each zinc atom having one to four carbon neighbors and the remaining being nitrogen atoms. It has one of the highest negative thermal expansion coefficients.
Chemical properties
Zn(CN)2 is insoluble in most solvents, as is typical for an inorganic polymer. To form anionic complexes, the solid dissolves in or is degraded by, aqueous solutions of basic ligands such as hydroxide, ammonia, and additional cyanide.
Applications
Electroplating
The main application of Zn(CN)2 is for electroplating of zinc from aqueous solutions containing additional cyanide.
Organic synthesis
Zn(CN)2 is used in the Gatterman reaction to introduce the formyl group into aromatic compounds, where it is a more convenient, safer, and non-gaseous alternative to HCN. Because the reaction employs HCl, Zn(CN)2 also provides the reaction with ZnCl2, a Lewis acid catalyst, in situ. Zn(CN)2 has been used in the synthesis of 2-hydroxy-1-naphthaldehyde and mesitaldehyde, for example.
Zn(CN)2 is also used as a catalyst for aldehyde and ketone cyanosilylation.