The lightest gas in the Universe is hydrogen. Under normal atmospheric pressure, one liter of this gas weighs only 90 mg, making it 11 times lighter than the air humans breathe. Liquid hydrogen (LH2) is the element hydrogen in its liquid state. Hydrogen exists in nature in the molecular H2 form.
Converting hydrogen gas to liquid hydrogen by cooling it to a very low temperature is a cutting-edge approach for storing maximal hydrogen in a limited volume. When hydrogen is cooled to a temperature less than -252,87 °C, it becomes a liquid. Liquid hydrogen has a density of approximately 71 kg/m3 at -252.87°C and 1.013 pressure. At this pressure, a 75-liter tank can hold 5 kilograms of hydrogen. Tanks must be completely segregated in order to keep liquid hydrogen at this temperature.
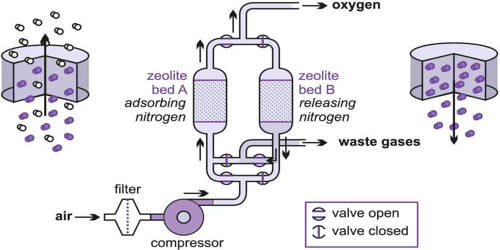
H2 must be cooled below its critical point of 33 K in order to exist as a liquid. However, in order for H2 to be totally liquid at atmospheric pressure, it must be cooled to 20.28 K (−252.87 °C; −423.17 °F). One common way of obtaining liquid hydrogen employs a compressor that, in both look and principle, resembles a jet engine. Liquid hydrogen is a concentrated form of hydrogen storage that is commonly employed. At normal temperature and pressure, storing a gas as a liquid takes up less space than storing it as a gas. However, when compared to other common fuels, the liquid density is extremely low. It can be kept as a liquid in pressurized and thermally insulated containers once liquefied.
There are two spin isomers of hydrogen; liquid hydrogen consists of 99.79% parahydrogen and 0.21% orthohydrogen.

Properties
When it burns with only oxygen, it produces water vapor (though when it burns with oxygen and nitrogen, it can produce toxic chemicals, which can be cooled with some liquid hydrogen. Because water is often thought to be environmentally friendly, an engine that burns it can be considered to have “zero emissions.” However, water vapor emitted in the atmosphere by aircraft contributes to global warming (to a lesser extent than CO2). The specific energy of liquid hydrogen is also substantially higher than that of gasoline, natural gas, or diesel.
Liquid hydrogen has a density of only 70.85 g/L (at 20 K), resulting in a relative density of only 0.07. Despite having more than double the specific energy of other fuels, it has a relatively low volumetric energy density, many orders of magnitude lower.
Uses
Liquid hydrogen is a typical liquid rocket fuel used in rocketry applications. Both NASA and the United States Air Force maintain a large number of liquid hydrogen tanks, each with a capacity of up to 3.8 million liters (1 million U.S. gallons). Most liquid hydrogen rocket engines cool the nozzle and other components before being mixed with the oxidizer — commonly liquid oxygen (LOX) and burned to create water with traces of ozone and hydrogen peroxide.
Liquid hydrogen can be used to power an internal combustion engine or a fuel cell. This type of hydrogen has been used to build submarines and concept hydrogen vehicles. Builders can occasionally alter and share equipment with systems developed for liquefied natural gas due to their resemblance (LNG). The hydrogen volumes required for burning, however, are significant due to the decreased volumetric energy.