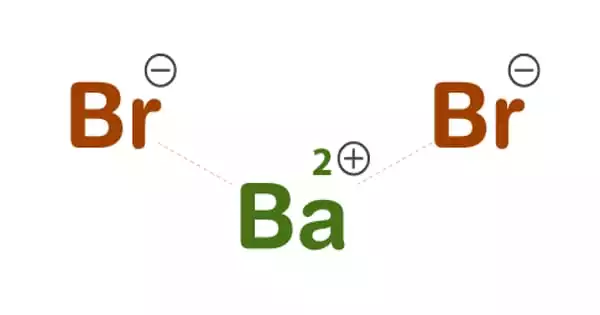
The chemical compound with the formula BaBr2 is barium bromide. It is metallic alkaline in nature, although it never appears pure. It is a chemical compound similar to barium chloride. Furthermore, this chemical dissolves readily in water and is hazardous. Furthermore, it is bromide of barium and bromine. It is also a precursor of chemicals that are used in photography. In addition, barium bromide is a precursor to other bromides. It dissolves well in water and is hazardous, just like barium chloride.
Barium bromide is a metallic alkaline element found on Earth. This combination is never found in its pure form in nature. This is due to barium bromide’s reactivity with air. Furthermore, barium bromide reacts with substances such as oxygen, sulfur, or carbon to generate barium compounds.
Properties
The barium bromide compound is a white crystalline powder. The exact mass of this inorganic compound is 297.74 grams per mole. However, the monoisotopic mass of BaBr2 is 297.742 gram per mole. It appears as a white solid which is soluble in water and is toxic in aqueous form.
- Molecular Weight: 297.14
- Appearance: White beads
- Melting Point: 857° C (1,575° F)
- Boiling Point: 1,835° C (3,335° F)
- Density: 4.78 g/cm3
- Solubility in H2O: N/A

Preparation
BaBr2 can be prepared from barium carbonate or barium sulfide by reacting with hydrobromic acid (HBr) to produce hydrated barium bromide. Barium bromide can be prepared by treating barium sulfide or barium carbonate with hydrobromic acid:
BaS + 2 HBr → BaBr2 + H2S
BaCO3 + 2 HBr → BaBr2 + CO2 + H2O
Barium bromide crystallizes from concentrated aqueous solution in its dihydrate , BaBr2·2H2O. Heating this dihydrate to 120 °C gives the anhydrous salt.
Reactions
The barium bromide solutions react with the salts of sulfate and produce a solid precipitate of barium sulfate:
BaBr2 + SO42- → BaSO4 + 2Br–
Barium bromide reacts with oxalic acid to produce solid precipitates of barium oxalate:
BaBr2 + C2O42- → BaC2O4 + 2Br–
It is a white odourless powder.
The reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride:
BaBr2 + F– → BaF2 + 2Br–
BaF2 is a colourless solid that occurs in nature.
The reaction between barium bromide and phosphoric acid produces solid precipitate that is, barium phosphate:
BaBr2 + PO43- → Ba3(PO4)2 + 2Br–
Uses
Barium bromide serves as a precursor to chemicals used in photography as well as other bromides. Historically, barium bromide was used to purify radium using Marie Curie’s fractional crystallization technique. Because radium preferentially precipitates in a solution of barium bromide, the radium-to-barium ratio in the precipitate would be greater than the ratio in the solution.
- It is used as a precursor in chemicals.
- Used in photography.
- It was used in a process called fractional crystallization to purify radium.
- Used in the manufacturing of other bromides.
- Used in the production of phosphors.
Safety
Barium bromide, like other water-soluble barium salts, is poisonous. There are various documented risks associated with the use of barium bromide. The inorganic substance barium can obstruct the entrance of intracellular potassium. As a result, potassium is transferred from the extracellular compartment to the intracellular compartment. There is a decrease in the resting membrane potential, which electrically unexcites the muscle fibers. As a result, it has the potential to cause paralysis. When barium bromide is ingested, it can cause serious poisoning in humans.