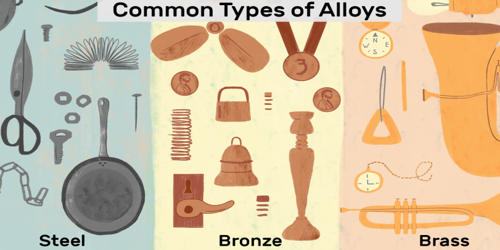
An alloy is a combination of metals or metals combined with one or more other elements. It is a substance made by melting two or more elements together, at least one of the metal. For example, combining the metallic elements gold and copper produces red gold, gold, and silver become white gold, and silver combined with copper produces sterling silver. The components of alloys cannot be separated using a physical means. Elemental iron, combined with non-metallic carbon or silicon, produces alloys called steel or silicon steel. Examples of alloys include stainless steel, brass, bronze, white gold, 14k gold, and sterling silver.
An alloy is homogeneous and retains the properties of a metal, even though it may include metalloids or nonmetals in its composition. They are usually synthetic materials, developed by scientists for special purposes. The resulting mixture forms a substance with properties that often differ from those of the pure metals, such as increased strength or hardness. They generally have especially desirable properties quite different from the metals from which they are made.
Alloys are used because their chemical and physical properties are superior for an application than that of the pure element components. They are used in a wide variety of applications, from the steel alloys, used in everything from buildings to automobiles to surgical tools, to exotic titanium-alloys used in the aerospace industry, to beryllium-copper alloys for non-sparking tools. In some cases, a combination of metals may reduce the overall cost of the material while preserving important properties. In other cases, the combination of metals imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength. Examples of alloys are steel, solder, brass, pewter, duralumin, bronze, and amalgams.
There are a wide variety of alloying elements that serve different purposes for different base materials.
- Chromium is a metal frequently used to help alloys resist corrosion.
- Nickel is a metal often added to materials to increase toughness.
- Copper is a metal used to make materials, such as aluminum, precipitation-hardenable.
Manganese is a metal usually alloyed to improve strength. - Lead is a metal alloying element that is used to improve machinability.
- Carbon is a nonmetal alloying element that is a necessary element to manufacture steel.
Alloys are defined by a metallic bonding character. The most common way to combine metals into an alloy is by melting them, mixing them together, and then allowing them to solidify and cool back to room temperature. The alloy constituents are usually measured by mass percentage for practical applications, and in atomic fraction for basic science studies. It is a substance composed of two or more metals, or of a metal or metals with a nonmetal, intimately mixed, as by fusion or electrodeposition. Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy. They can be further classified as homogeneous, or heterogeneous or intermetallic.