Lead from metal-organic perovskite compounds is particularly easily absorbed by plants. The bioavailability of lead from inorganic compounds, such as those found in batteries, is significantly higher. Certain perovskite compounds are viewed as a great hope for better and, more importantly, less expensive solar cells. Unfortunately, the best perovskite solar cells contain toxic lead, posing an environmental risk. However, replacing lead with less toxic elements is surprisingly difficult.
Perovskite solar cells, while a promising solution for capturing solar energy, contain lead, which is toxic to the environment and a serious health hazard. Scientists have now discovered a very elegant and efficient solution by adding a transparent phosphate salt that does not interfere with light-conversion efficiency while also preventing lead from seeping into the soil in the event of a solar panel failure.
“The solar energy-to-electricity conversion of perovskite solar cells is incredible, around 25%, and is now approaching the performance of the best silicon solar cells,” says Professor László Forró of EPFL’s School of Basic Sciences. “However, their main component is lead, which is poisonous; if the solar panel fails, it can wash into the soil, enter the food chain, and cause serious diseases.”
Scientists have now found a very elegant and efficient solution by adding a transparent phosphate salt that doesn’t interfere with light-conversion efficiency while preventing lead from seeping into the soil in cases of solar panel failure.
The issue is that lead can dissolve in water in the majority of halide perovskites. This water solubility and solubility in other solvents is actually a great advantage, as it simplifies and lowers the cost of building perovskite solar panels – another benefit in addition to their performance. However, lead’s water solubility can pose a serious environmental and health risk when the panel breaks or becomes wet, such as when it rains.
As a result, the lead must be captured before it enters the soil, and it must be recyclable. This issue has sparked extensive research because it is the primary impediment to regulatory authorities approving large-scale commercial production of perovskite solar cells. Attempts to synthesize non-water-soluble and lead-free perovskites, on the other hand, have yielded poor results.
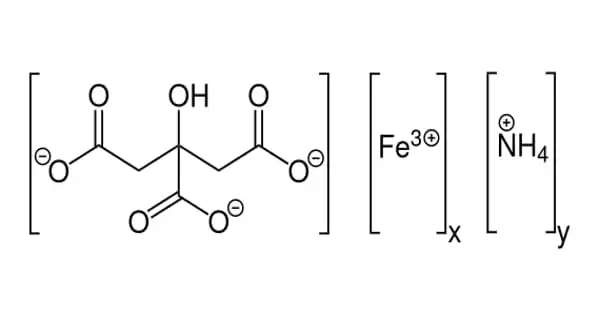
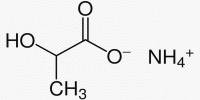
Now, Forró’s team has devised an elegant and efficient solution that involves the use of a transparent phosphate salt that does not block solar light and thus has no effect on performance. In the event that the solar panel fails, the phosphate salt immediately reacts with lead to form a water-insoluble compound that cannot leach into the soil and can be recycled. The study was published in the journal ACS Applied Materials & Interfaces.
“We discovered a few years ago that cheap and transparent phosphate salt crystals, such as those found in soil fertilizers, can be incorporated into various parts of sandwich-like lead halide perovskite devices, such as photodetectors, LEDs, or solar cells,” says Endre Horváth, the study’s first author. “In the presence of water, these salts react instantly with lead ions and precipitate them into extremely non-water-soluble lead phosphates.”
“The ‘fail-safe’ chemistry prevents lead ions from leaching out and can make perovskite devices safer to use in the environment or near humans,” says Márton Kollár, the chemist behind perovskite crystal growth.
“We demonstrate that this approach can be used to build functional photodetectors, and we propose that the broad community of researchers and R&D centers working on various devices such as solar cells and light-emitting diodes implement it in their respective prototypes,” says Pavao Andrievic, who characterized the sensitive photodetectors.
Certain compositions of perovskite solar cells can convert ultraviolet and visible light into electricity very efficiently, suggesting that they could be excellent hybrid-tandem partners for absorber materials that efficiently convert infrared light, such as crystalline silicon. “This is an extremely important study, I would say a central one, for large-scale commercialization of perovskite-based solar cells,” Forró concludes.
A perovskite solar cell (PSC) is a type of solar cell in which the light-harvesting active layer is a perovskite-structured compound, most commonly a hybrid organic-inorganic lead or tin halide-based material. However, when exposed to continuous illumination, moisture, and high temperatures, the unstable nature of perovskite was observed, impeding commercial development in the long run and thus becoming the main issue that needs to be resolved urgently.