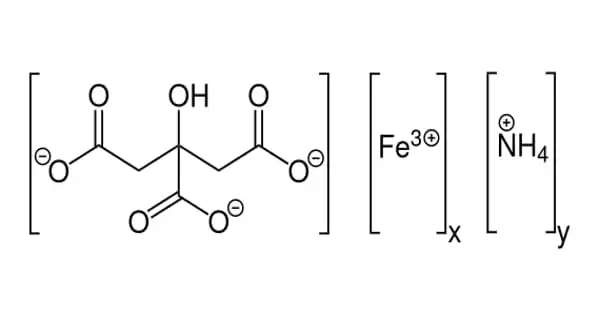
The formula for ammonium ferric citrate is (NH4)5[Fe(C6H4O7)2]. It’s a reddish-brown to crimson solid with a subtle ammonia odor. It dissolves in water. This chemical is distinguished by the fact that it is very soluble in water, as opposed to ferric citrate, which is not.
Each component of citric acid has lost four protons in its crystal structure. The ferric center is ligated by the deprotonated hydroxyl group and two of the carboxylate groups, while the third carboxylate group binds with the ammonium. The most serious danger is the harm to the ecosystem. Immediate action should be done to limit its environmental spread. It is used in medicine, construction, and as a feed ingredient.
Properties
Ferric ammonium citrate is a reddish-brown to crimson solid with an ammonia-like odor. It dissolves in water. Ferric ammonium citrate is a combination of undefined composition that contains ferric and ammonium cations and citrate(3-) anions. It can be formed as red crystals, brownish-yellow powder, or green crystals or powder. It is used as an acidity regulator and anticaking agent in foods. It is also used as a positive oral contrast agent in magnetic resonance imaging and was previously given orally as an iron source to treat iron deficiency anaemia.
- Molecular Weight: 261.975
- Appearance: Green powder or granules
- Melting Point: N/A
- Boiling Point: N/A
- Density: 1.8 g/cm3
- Solubility in H2O: 1200 g/L (20 °C)

Production method
The ferric hydroxide is dissolved in citric acid, neutralized with ammonia solution, followed by drying to obtain it.
(1) Creating ferric hydroxide After adding ferrous sulfate to the water, sulfuric acid was progressively added while stirring, followed by the addition of an aqueous sodium chlorate solution. Then, aggressively mix and raise the temperature to above 80 °C before adding sodium chlorate. To obtain the ferric sulfate solution, stir until the reaction is complete (no more ferrous reaction as shown by the ferricyanide test). Add this solution to the reaction tank, then add the sodium hydroxide solution and rapidly agitate with the reaction temperature set to 80-90 °; when the reaction solution changes from viscous to thin, add water for washing until the sulfate and chlorine levels are met. To obtain iron hydroxide, allow to dry.
(2) Preparation of ferric ammonium citrate; add citrate, ferric hydroxide, and water to the reaction tank, stir, and maintain the temperature at 95 °C above for 1 hour. The mixture was then chilled to 50°C before being immersed in ammonia with stirring. Stay for more than 48 hours. To obtain ferric ammonium citrate, the supernatant was filtered, then the filtrate was concentrated to a paste and dried at 80 ° C. Ferrous sulfate has a total output of 73-75 percent.
Uses
Ammonium ferric citrate is a food additive used as an acidity regulator. Other uses for ammonium ferric citrate include water purification and printing. It is used as a reducing agent of metal salts of low activity like gold and silver and is also in a commonly used recipe with potassium ferricyanide to make cyanotype prints
Ammonium ferric citrate has a range of uses, including:
- It has an INS number as a food ingredient and is used as an acidity regulator. It is most famous for its use in the Scottish beverage Irn-Bru.
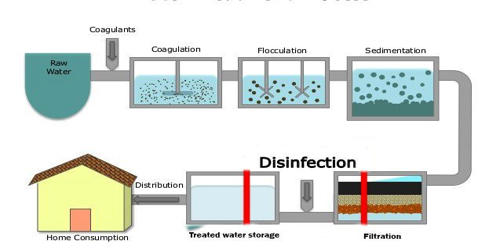
- Purification of water.
- As a reducing agent for low activity metal salts such as gold and silver.
- As part of the cyanotype photographic process, potassium ferricyanide was used.
- Used in the Kligler’s Iron Agar (KIA) test to detect enterobacteriaceae bacteria by watching their metabolism of various carbohydrates, which results in the production of hydrogen sulfide.
- Ammonium ferric citrate is employed as a contrast medium in medical imaging.
- Used as a hematinic.