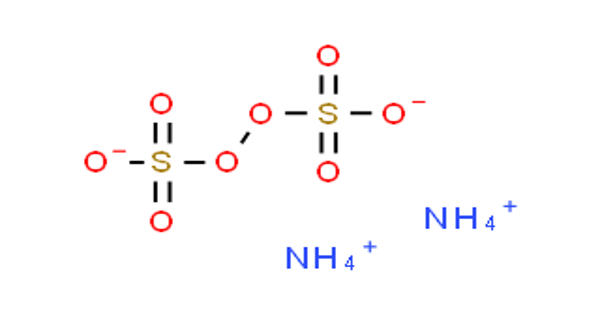
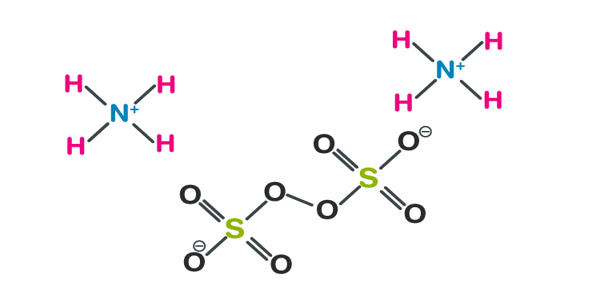
Ammonium persulfate (APS) is the inorganic compound with the formula (NH4)2S2O8. It appears as a white crystalline solid. It is a colorless (white) salt that is highly soluble in water, much more so than the related potassium salt. It is an oxidizing agent that is used with TEMED to catalyze the polymerization of acrylamide and bisacrylamide to prepare polyacrylamide gels for electrophoresis. It is a strong oxidizing agent that is used in polymer chemistry, as an etchant, and as a cleaning and bleaching agent. It does not burn readily but may cause spontaneous ignition of organic materials.
Properties
The dissolution of the salt in water is an endothermic process. It is an oxidizing agent that is often used with tetramethylethylenediamine to catalyze the polymerization of acrylamide and bisacrylamide to prepare polyacrylamide gels for electrophoresis. It is a colorless salt which is highly soluble in water, much more than the related potassium salt. It is a strong oxidizing agent which is used in polymer chemistry, as a cleaning and bleaching agent, and as an etchant.
- Melting point: 120 °C
- Density: 1.98
- Vapor density: 7.9 (vs air)
- Solubility H2O: soluble
- Form: powder
- Color: White to yellow
- Specific Gravity: 1.982
- Odor: Odorless.
Preparation
Ammonium persulfate is prepared by electrolysis of a cold concentrated solution of either ammonium sulfate or ammonium bisulfate in sulfuric acid at a high current density. It is prepared by using the method of electrolysis of a cold concentrated solution of either of these substances: ammonium sulfate or ammonium bisulfate, in the presence of the sulfuric acid at a very high current density. The method was first described by Hugh Marshall.
Uses
As an oxidizing agent and a source of radicals, APS finds many commercial applications.
It is readily soluble in water and is a strong oxidizing agent. It has a variety of applications in polymer chemistry, such as a bleaching agent, cleaning agent, and an etchant.
Commercially important polymers prepared using persulfates include styrene-butadiene rubber and polytetrafluoroethylene. In solution, the dianion dissociates to give radicals:
[O3SO–OSO3]2- ⇌ 2 [SO4]–
Illustrative of its powerful oxidizing properties, it is used to etch copper on printed circuit boards as an alternative to ferric chloride solution. This property was discovered many years ago. In 1908, John William Turrentine used a dilute ammonium persulfate solution to etch copper.
Ammonium persulfate is a standard ingredient in hair bleach.
Persulfates are used as oxidants in organic chemistry. For example, in the Minisci reaction.
Safety
Airborne dust containing ammonium persulfate may be irritating to eye, nose, throat, lung and skin upon contact. Exposure to high levels of dust may cause difficulty in breathing.
Information Source: