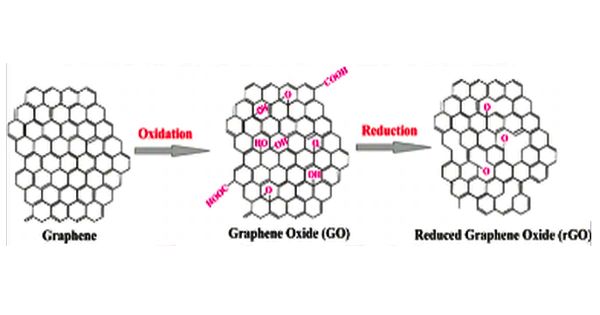
Graphite oxides contain oxygen and hydroxide radicals in various amounts, which make covalent bonds with carbon and, as a consequence, a layer of carbon atoms is not flat. It formerly called graphitic oxide or graphitic acid is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers. It is easy to process since it is dispersible in water and other solvents. Due to the oxygen in its lattice graphene oxide is not conductive, but it can be reduced to graphene by chemical methods. Graphene oxide is not a good conductor, but processes exist to augment its properties. It is commonly sold in powder form, dispersed, or as a coating on substrates.
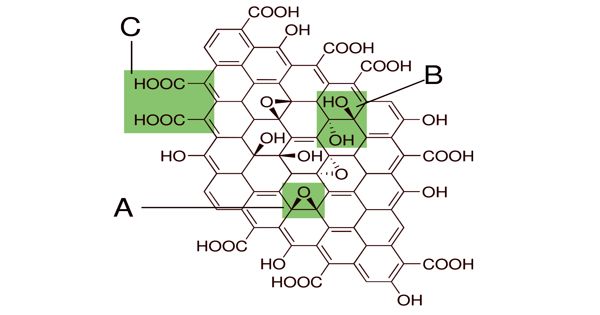
Graphene oxide (GO) is a unique material that can be viewed as a single monomolecular layer of graphite with various oxygen-containing functionalities such as epoxide, carbonyl, carboxyl, and hydroxyl groups.
Graphite oxide exhibits an identical layered structure to graphite, but it contains a huge amount of oxygen-containing groups, which results in an increase in the interlayer spacing and makes the atomic-thick layers hydrophilic as well. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.
The bulk material spontaneously disperses in basic solutions or can be dispersed by sonication in polar solvents to yield monomolecular sheets, known as graphene oxide by analogy to graphene, the single-layer form of graphite. The problem with this technique is the quality of graphene sheets produced, which (with certain methods) displays properties currently below the theoretical potential of pristine graphene compared to other methods such as mechanical exfoliation.
Graphene oxide sheets have been used to prepare strong paper-like materials, membranes, thin films, and composite materials. Functionalization of graphene oxide can fundamentally change graphene oxide’s properties. Initially, graphene oxide attracted substantial interest as a possible intermediate for the manufacture of graphene. The resulting chemically modified graphenes could then potentially become much more adaptable for a lot of applications.
There are many ways in which graphene oxide can be functionalized, depending on the desired application. For optoelectronics, biodevices or as a drug-delivery material, for example, it is possible to substitute amines for the organic covalent functionalization of graphene to increase the dispersability of chemically modified graphenes in organic solvents.
One of the main advantages of graphene oxide is that it is dispersible to water. This makes it possible to use solution-based processes. The graphene obtained by reduction of graphene oxide still has many chemical and structural defects which are a problem for some applications but an advantage for some others. Another benefit of graphene oxide is that it can be reduced to graphene by using chemical, thermal or electrochemical methods.
Information Source: