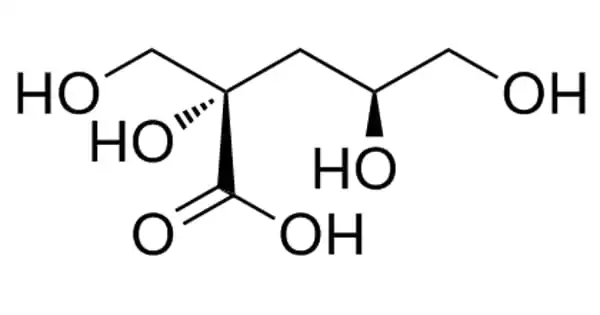
Isosaccharinic acid (ISA) is a six-carbon sugar-acid formed by calcium hydroxide reacting with lactose and other carbohydrates. It is of interest because it can form in intermediate-level nuclear waste stores when cellulose is degraded by the calcium hydroxide found in cement-like Portland cement. In cold water, the calcium salt of the alpha form of ISA is very crystalline and insoluble, but it is soluble in hot water.
Properties
- Boiling point: 583.8±50.0 °C (Predicted)
- Density: 1.599±0.06 g/cm3 (Predicted)
- Melting point: 189-194 °C (Solv: water (7732-18-5); acetone (67-64-1))
- Vapour Pressure: 0.0±3.7 mmHg at 25°C
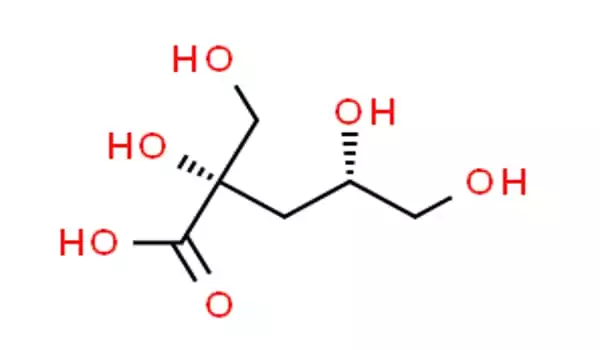
Two of the three steps in the formation of ISA are thought to be catalyzed by calcium ions acting as Lewis acids. The first step is likely to be a rearrangement of the reducing sugar end of the cellulose (or lactose) into a keto sugar, and the second step is likely to be a reaction similar to the base-catalyzed dehydration that often occurs after an aldol reaction. In this second step, an alkoxide (derived from sugar) serves as the hydroxide leaving group; this second step is unlikely to require the calcium’s Lewis acidity. The final step is a benzilic acid rearrangement from a 1,2-diketone (1,5,6-trihydroxyhexane-2,3-dione) formed from the carbohydrate.
Sugars dehydrate to form furans such as furfural and 5-hydroxymethylfurfural under acidic conditions. Nuclear waste is produced as a result of nuclear power plant energy production and the use of radioactive materials for military, industrial, and medical applications. Nuclear waste will be stored in deep geological facilities to ensure its safe isolation from the biosphere and to reduce the potential risk of exposure to harmful compounds to humans and the environment.
By forming an ester between the carboxylic acid group and one of the alcohols, the acid tends to form a 5-membered ring (lactone) in acidic solutions. When treated anhydrous with acetone, an acid, and a dehydration agent, two of the alcohol groups can be protected as cyclic acetone acetals, leaving only one alcohol. Prolonged treatment with 2,2-dimethoxypropane forms a protected form of ISA in which all four alcohol groups are protected as acetone acetals and the carboxylic acid is in the form of the methyl ester. These protected forms of ISA have been used as starting materials for chiral organic compounds such as anthracyclines.