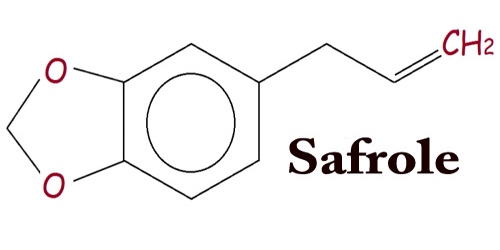
Safrole (CH2O2C6H3CH2CH=CH2) appears as a clear colorless or slightly yellow liquid with the odor of sassafras. It is a phenylpropene that is typically extracted in the form of sassafras oil from the root bark or the fruit of sassafras plants or is synthesized from catechol or other similar methyleneedioxy compounds. Tiny amounts are present in a wide range of plants, where it functions as a natural antifeed. The main source of safrole includes Ocotea cymbarum oil made up of Ocotea pretiosa (a plant growing in Brazil), and Sassafras albidum, which grows in eastern North America, are the most natural sources of safrole. It has a characteristic “sweet-shop” aroma.
Safrole is that the key component of brown oil, and also exists in small amounts during a wide range of plants, where it functions as a natural pesticide. It’s a precursor within the synthesis of the insecticide synergist piperonyl butoxide, the fragrance piperonal via isosafrole, and therefore the empathogenic/entactogenic drug MDMA. It’s a member of the methylenedioxybenzene group of compounds, many of which (e. g. piperonyl butoxide) are extensively used as insecticide synergists.
Safrole may be a major source of human exposure to safrole is thru consumption of spices, like nutmeg, cinnamon, and black pepper, within which safrole could be a constituent. It’s one amongst the natural components of refined oils in additional than fifty types of vegetables. Many of them is made into seasonings and are one in all the most important components of essential oils, like sassafras, camphor, nutmeg, black pepper, sweet basil, mace, cinnamon, anise and piper betle flower.
Safrole remained to be determined whether the molecule’s C3H5 group was a propenyl group (R-CH=CH-CH3) or an allyl group (R-CH2-CH=CH2). Safrole and isosafrole (S-isomer of safrole) were once used extensively as a seasoning in soft drinks. Since safrole and isosafrole are carcinogens, adding oil in soft drinks has been prohibited within the U.S. (United States) since 1970, while it absolutely was defined as a sort of artificial additive and treated as a special element of seasonings within the Republic of China with limited usage.
Safrole is additionally present in soft drink and has been used as an additive in chewing gum, toothpaste, soaps, and certain pharmaceutical preparations. It’s a weak hepatocarcinogen and it’s a matter of considerable interest whether the ally1 moiety or the methylenedioxy group or both, are involved within the mechanism of its carcinogenesis. it’s the principal component of brown camphor (Ocotea cymbarum) oil made of Ocotea pretiosa, a plant growing in Brazil, and oil made of Sassafras albidum.
In 2009, safrole was manufactured in the United States by just one facility worldwide and was available from 11 U.S. suppliers (ChemSources 2009). In 1980 U.S. safrole imports were 36,000 lb (HSDB 2009) and ranged from 11,000 to 132,000 lb in 1997, 2003, 2004 and 2005 (USITC 2009). In 1996, US exports of safrole totalled 6,600 lb and in 1998, 35,200 lb. Safrole is a versatile precursor to a great number of compounds. Examples include N-acylarylhydrazones, isosters, derivatives of aryl-sulfonamide, acidic derivatives of sulfonylhydrazone, benzothiazine derivatives, and many more.
Safrole is extensively metabolized, giving rise to an outsized number of metabolites. Metabolism involves essentially two major routes, oxidation of the ally1 side chain, and oxidation of the methylenedioxy group with subsequent cleavage to make the catechol. Safrole will be used as a flavoring agent in drugs and within the manufacture of heliotropin, perfumes, soaps, and piperonyl butoxide; it’s also been used as a preservative in mucilage and adhesive material and as a flotation frother. Studies within the 1960s suggested that safrole was carcinogenic, causing permanent liver damage in animals. Consequently, the US Food and Drug Administration (FDA) banned sassafras and safrole for human consumption.
The major toxicity of safrole and isosafrole comes from their carcinogenic nature after oxidation. Denser than water (density 1.09 g / cm3) and insoluble in water. Many mammals oxidize Safrole into1-hydroxysafrole, the derivatives of which include isosafrole and dihydrosafrole, both carcinogenic. Additionally, safrole oxide, a safrole metabolite, has an adverse effect on the central nervous system. Safrole oxide inhibits the expression of integrin β4/SOD, which leads to apoptosis of the nerve cells.
Information Sources: