Lithium aluminate (LiAlO2), also known as lithium aluminum oxide, is an inorganic chemical compound that is a lithium aluminate. Lithium aluminate is used as a lattice matching substrate for gallium nitride in microelectronics. Lithium aluminate is of interest in nuclear technology as a solid tritium breeder material for preparing tritium fuel for nuclear fusion.
Properties
- Compound Formula: AlLiO2
- Molecular Weight: 65.921
- Appearance: White crystalline powder or solid in various forms such as rod
- Melting Point: 1625 °C
- Boiling Point: N/A
- Density: 2.615 g/cm3
- Solubility in H2O: Insoluble
- Exact Mass: 65.987372
- Monoisotopic Mass: 65.987372
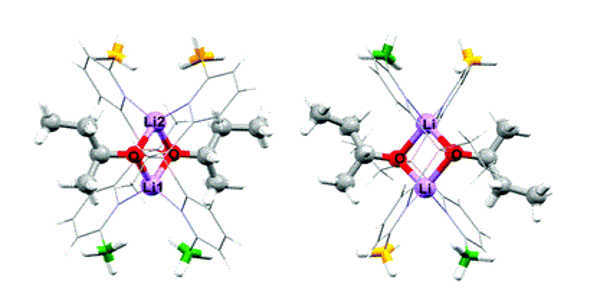
Structure
Lithium aluminate is a layered double hydroxide (LDH) with the same crystal structure as hydrotalcite. At high pH (12.5 – 13.5), lithium aluminate solubility is much lower than that of aluminum oxides. Lithium nitrate is sometimes used as an additive to cement in the conditioning of low- and intermediate-level radioactive waste (LILW) to reduce aluminum corrosion at high pH and subsequent hydrogen production. When lithium nitrate is added to cement, a passive layer of LiH(AlO2)2 •5 H2O forms on the surface of metallic aluminum waste immobilized in mortar.
The lithium aluminate layer, which is also a pore filler, is insoluble in cement pore water and protects the underlying aluminum oxide covering the metallic aluminum from dissolution at high pH. This inhibits the oxidation of aluminum by water protons and reduces the rate of hydrogen evolution by a factor of ten.
Lithium aluminate also finds its use as an inert electrolyte support material in molten carbonate fuel cells, where the electrolyte may be a mixture of lithium carbonate, potassium carbonate, and sodium carbonate. Lithium aluminate is of interest in nuclear technology as a solid tritium breeder material for preparing tritium fuel for nuclear fusion.
Although solid-state reactions have been used to create lithium aluminate polymorphs, these ceramics typically exhibit limitations due to a lack of control over particle size, morphology, and specific surface area.
In molten carbonate fuel cells, lithium aluminate is used as an inert electrolyte support material, where the electrolyte may be a mixture of lithium carbonate, potassium carbonate, and sodium carbonate.