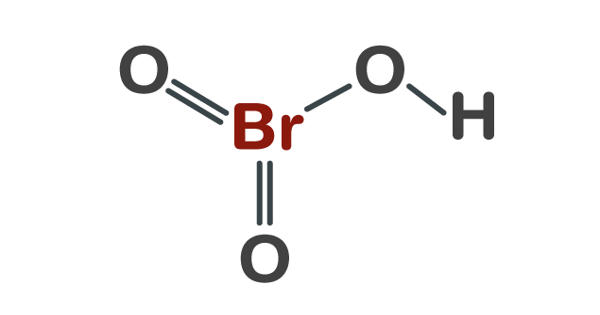
Bromine is a chemical element with the symbol Br and atomic number 35. It is a deep red noxious liquid and a member of the halogen elements, or Group 17 (Group VIIa) of the periodic table. It is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly colored vapor. It is the 44th most common element in Earth’s crust, according to Periodic Table with an abundance of 2.4 parts per million by weight. Its properties are intermediate between those of chlorine and iodine.
Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard in the residues (bitterns) from the manufacture of sea salt at Montpellier. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek βρῶμος (“stench”), referring to its sharp and disagreeable smell.
Properties
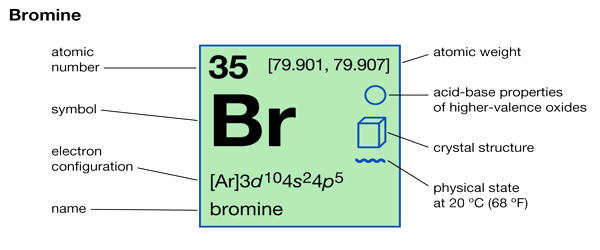
- Atomic number: 35
- Atomic mass: 79.904 g.mol -1
- Electronegativity according to Pauling: 2.8
- Density: 3.1 g.cm-3 at 20°C
- Melting point: – 7.2 °C
- Boiling point: 58.8 °C
- Vanderwaals radius: 0.165 nm
- Ionic radius: 0.195 nm (-1)
- Isotopes: 10
- Electronic shell: [ Ar ] 3d10 4s2 4p5
- Energy of first ionization: 1142.7 kJ.mol -1
Elemental bromine is very reactive and thus does not occur free in nature but in colorless soluble crystalline mineral halide salts, analogous to table salt. It is the only nonmetallic element that is liquid under ordinary conditions, it evaporates easily at standard temperature and pressures in a red vapor that has a strong disagreeable odor resembling that of chlorine. While it is rather rare in the Earth’s crust, the high solubility of the bromide ion (Br−) has caused its accumulation in the oceans. It is very harmful to the atmosphere. Bromine atoms are 40 to 100 times more destructive in the ozone layer than chlorine atoms.
Occurrences
Bromine occurs in compounds present in seawater, natural brines and salt-lake evaporates. Commercially the element is easily extracted from brine pools, mostly in the United States, Israel, and China. Bromine mineral deposits in the United States are in natural brine wells in Michigan and Arkansas. The mass of bromine in the oceans is about one three-hundredth that of chlorine. Worldwide production estimated to be around 330,000 tons per year. It is also recovered in Israel, Russia, France, and Japan, according to the Minerals Education Coalition. About 30% of the bromine in the atmosphere comes from human activities, the rest is natural.
Uses
Bromine is used in many areas such as agricultural chemicals, dyestuffs, insecticides, pharmaceuticals, and chemical intermediates. It is used to purify water, in medicines, and as sanitizers. Some uses are being phased out for environmental reasons, but new uses continue to be found.
Bromine compounds can be used as flame retardants. They are added to furniture foam, plastic casings for electronics and textiles to make them less flammable.
However, the use of bromine as a flame retardant has been phased out in the USA because of toxicity concerns. It is hazardous. It is corrosive to human tissue in its liquid state, and irritates eyes and the throat, and is highly toxic when inhaled in a vapor state.
Information Source: