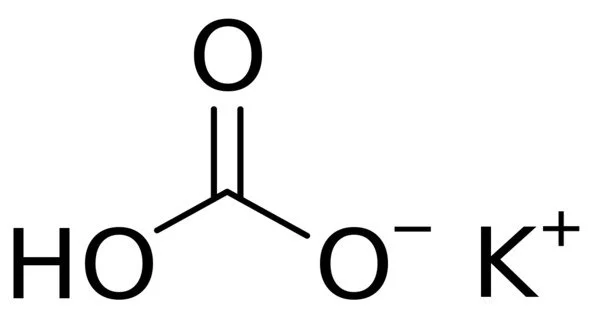
Potassium bicarbonate is an inorganic compound with the chemical formula KHCO3. It’s a solid white color. It is a potassium salt that is the monopotassium salt of carbonic acid. It has fungicidal properties and is used in organic farming to control powdery mildew and apple scab. It functions as a food acidity regulator, a raising agent, a buffer, and an antifungal agrochemical. It is a potassium salt and an organic salt. It contains hydrogencarbonate.
It is used in medicine as an antacid. It is used to prevent and treat low blood potassium, and it must be carefully monitored in patients with chronic renal disease or any condition that impairs potassium excretion.
Properties
Potassium bicarbonate is a white, crystalline, slightly alkaline and salty substance. It is produced by the passage of carbon dioxide through an aqueous potassium carbonate solution.
- Chemical formula: KHCO3
- Molar mass: 100.115 g/mol
- Appearance: white crystals
- Odor: odorless
- Density: 2.17 g/cm3
- Melting point: 292 °C (558 °F; 565 K) (decomposes)
- Solubility in water: 22.4 g/100 mL (20 °C)
- Solubility: practically insoluble in alcohol

Production and reactivity
It is manufactured by treating an aqueous solution of potassium carbonate with carbon dioxide:
K2CO3 + CO2 + H2O → 2 KHCO3
Decomposition of the bicarbonate occurs between 100 and 120 °C (212 and 248 °F):
2 KHCO3 → K2CO3 + CO2 + H2O
This reaction is employed to prepare high purity potassium carbonate.
Preparation
When an aqueous solution of K2CO3 (potassium carbonate) is treated with carbon dioxide gas, KHCO3 is formed. The chemical equation for this reaction is given by:
CO2 + K2CO3 + H2O → 2KHCO3
The standard enthalpy of formation for this compound corresponds to -963.2 kilojoules per mole. When heated to 120oC, potassium bicarbonate begins to undergo a decomposition reaction to yield water, carbon dioxide, and potassium carbonate.
Uses
- Food and drink
This compound provides carbon dioxide for baking leavening. For those on a low-sodium diet, it can be used in place of baking soda (sodium bicarbonate), and it is an ingredient in low-sodium baking powders. It is widely used in a variety of applications as an inexpensive, nontoxic base to regulate pH or as a reagent. Examples include use as a buffering agent in medications and as a winemaking additive. Potassium bicarbonate is frequently added to club soda to improve taste and soften the effervescence effect.
- Fire extinguishers
Potassium bicarbonate is used as a fire suppression agent (“BC dry chemical”) in some dry chemical fire extinguishers, as the main component of the Purple-K dry chemical, and in some applications of condensed aerosol fire suppression. It is the only dry chemical fire suppression agent approved by the National Fire Protection Association for use at airport crash rescue sites. It is approximately twice as effective as sodium bicarbonate in fire suppression.
- Agriculture
Potassium bicarbonate is widely used in agriculture, particularly for neutralizing acidic soil. It is a fungicide that is effective against powdery mildew and apple scab and is approved for use in organic farming. When mixed 1/4 cup per gallon, potassium bicarbonate is a contact killer for Spanish moss.