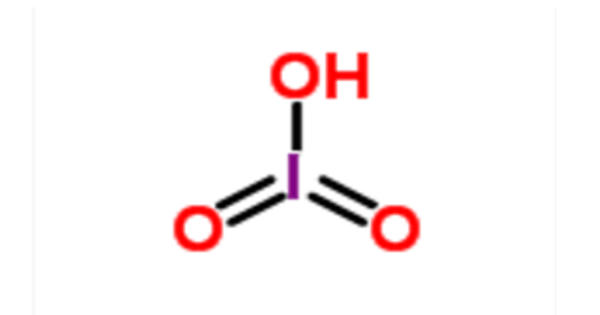
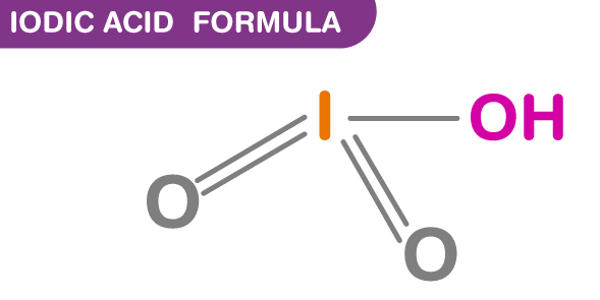
Iodic acid (HIO3) is an iodine oxoacid. It is a white water-soluble solid. It has a role as an astringent. Its robustness contrasts with the instability of chloric acid and bromic acid. It is the conjugate acid of an iodate. It is also known as monoiodic acid, trioxoiodic acid, hydrogen trioxoiodate, hydroxidodioxidoiodine, and iodic(V) acid.
Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. It contains iodine in the oxidation state +5 and it is one of the most stable oxo-acids of the halogens in its pure state. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.
Preparation
Iodic acid is soluble in water and also exists in a pure state. It can be produced by oxidizing iodine I2 with strong oxidizers such as nitric acid HNO3, chlorine Cl2, chloric acid HClO3 or hydrogen peroxide H2O2, for example:
I2 + 6 H2O + 5 Cl2 ⇌ 2 HIO3 + 10 HCl
Iodic acid can be obtained by oxidizing iodine with strong oxidizers like nitric acid, hydrogen peroxide, chloric acid and chlorine.
Properties
Iodic acid is a relatively strong acid with a pKa of 0.75. It is strongly oxidizing in acidic solution, less so in basic solution. When iodic acid acts as oxidizer, then the product of the reaction is either iodine, or iodide ion. Under some special conditions, iodic acid is reduced to iodine trichloride, a golden yellow compound in solution and no further reduction occurs.
- Molecular formula: HIO3
- Molecular mass: 175.909 g/mol
- Melting point: 110℃
- Density: 4.629 g/cm3
- Appearance: White to off-white powder, crystals, or chunks
- Boiling Point: N/A
In the absence of chloride ions, when there is an excess amount of reductant, then all iodate is converted to iodide ion. When there is an excess amount of iodate, then part of the iodate is converted to iodine. It may be used in preparation of ionization to form alkyl halides.
Uses
- Iodic acid is used as a strong acid in analytical chemistry.
- It is used in the salt industry to synthesize sodium and potassium iodate to increase the iodine content in the salt.
- It may be used to standardize solutions of both weak and strong bases, using methyl red or methyl orange as the indicator.
- In analytical chemistry, it is used as a strong acid.
- It is used as a standardized solution for both weak and the strong acid.
Information Source: