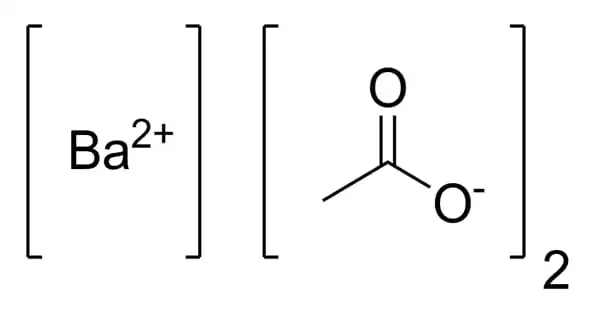
Barium acetate, having the chemical formula C4H6BaO4, is a salt of barium and acetic acid. It is a barium(II) and acetic acid salt. It’s a white powder that’s extremely soluble in water. It is typically formed through the interaction of acetic acid with barium carbonate. Although barium acetate is harmful to people, it has applications in chemistry and industry.
Properties
Barium acetate is a white powder that is very soluble: 55.8 g of barium acetate will dissolve in 100 g of water at 0°C. It has the appearance of white crystals or granules. When heated, it decomposes into barium carbonate. Furthermore, barium acetate is quite soluble in water. Furthermore, at a temperature of 20°C, its solubility in water is g/100ml: 59. The density of Barium acetate is 2.47 g/cm3. When heated, this salt decomposes into barium carbonate.
- Appearance: White Solid
- Melting point: 450 °C
- Density: 2.468 g/cm3
- Molar Mass: 255.43 g/mol
- Solubility in Water: Soluble

Preparation
Barium acetate decomposes to carbonate when heated in air. The other name of barium acetate is barium diacetate. It is generally produced by the reaction of acetic acid with barium carbonate:
BaCO3 + 2 CH3COOH → (CH3COO)2Ba + CO2 + H2O
The reaction is performed in solution and the barium acetate crystalizes out at temperatures above 41 °C. Between 25 and 40 °C, the monohydrate version crystalizes. Alternatively, barium sulfide can be used:
BaS + 2 CH3COOH → (CH3COO)2Ba + H2S
Again, the solvent is evaporated off and the barium acetate crystallized.
Reactions
Barium acetate decomposes to carbonate when heated in air. It interacts with acids, producing sulfate, chloride, and nitrate in the presence of sulfuric acid, hydrochloric acid, and nitric acid, respectively.
Uses
Barium acetate is used as a mordant in textile printing, as a drying agent in paints and varnishes, and as a lubricant in oil. It is used in chemistry to make additional acetates and as a catalyst in chemical synthesis.
For printing textile materials, experts employ barium acetate as a mordant. Furthermore, barium acetate is an excellent paint drying agent. Furthermore, this salt is useful in lubricating oil. In the discipline of chemistry, barium acetate is used in the manufacture of various acetates.
Health Hazards
If you swallow or inhale barium acetate, it can be dangerous. Additionally, this salt might irritate the eyes, skin, and respiratory tract. Furthermore, the lungs, heart, muscles, gastrointestinal system, and nerves are among its target organs. Above all, if an individual consumes barium acetate, it can be fatal. In addition, the barium ion is a muscle toxin. As a result, it can cause muscle activation and, eventually, paralysis.