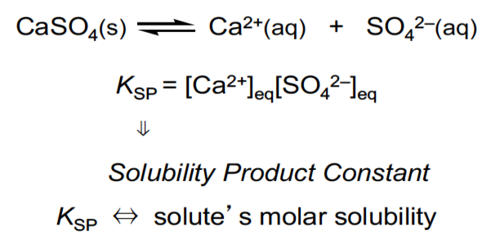Solubility equilibrium is the equilibrium associated with dissolving solids in water to form aqueous solutions. It is a type of dynamic equilibrium that exists when a chemical compound in the solid-state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solvent, such as acid or alkali. Each type of equilibrium is characterized by a temperature-dependent equilibrium constant. At the point where no more solid can dissolve, the solution is saturated. The solubility product constant is an equilibrium constant used in solubility equilibrium.
Solubility equilibria are important in pharmaceutical, environmental, and many other scenarios. The Solubility Equilibria are based on the assumption that the solids dissolve in water in order to give the basic particles from which they are formed.
Each of the molecular solids dissolves to give an individual aqueous molecule such as:
C12H22O11(S) ———(H2O)——> C12H22O11 (aq)
Overview
A solubility equilibrium exists when a chemical compound in the solid-state is in chemical equilibrium with a solution of that compound. The equilibrium is an example of dynamic equilibrium in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution and precipitation are equal to one another. When equilibrium is established, the solution is said to be saturated. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium between the pure solid and a solution of its ions.
Solubility equilibrium is base on the assumption that solids dissolve in water to give the basic particles from which they are formed. Solubility is temperature-dependent. A solution containing a higher concentration of solute than the solubility is said to be supersaturated. The equilibrium solubility of an additive in a polymer is the most important single physical property of the system since it determines how much additive can exist in the polymer as a homogeneous solution. A supersaturated solution may be induced to come to equilibrium by the addition of a “seed” which may be a tiny crystal of the solute, or a tiny solid particle, which initiates precipitation. Solubility products are determined experimentally by directly measuring either the concentration of one of the component ions or the solubility of the compound in a given amount of water. The solubility of any compound is given by the enthalpy change in the solution. The solubility of any ionic compounds in water is given by the ionization constant at equilibrium; here it refers to the non-Changement in the ionization constant of water.
There are three main types of solubility equilibria.
- Simple dissolution.
- Dissolution with dissociation reaction.
- Dissolution with ionization reaction.