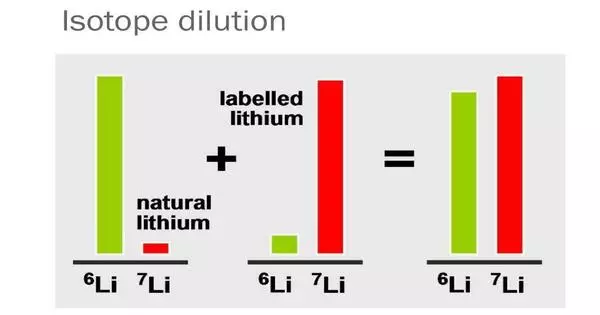
Isotope dilution analysis is a technique for estimating the quantity of chemical compounds. It is a technique used in analytical chemistry to determine the concentration of an element in a sample. In its most basic form, the procedure of isotope dilution entails adding known amounts of isotopically enriched substance to the tested sample. This method includes adding a known amount of a stable isotope of the element of interest (the “spike”) to a sample containing an unknown concentration of the element.
Mixing the isotopic standard with the sample effectively “dilutes” the standard’s isotopic enrichment, which serves as the foundation for the isotope dilution process. The element concentration can be reliably estimated by measuring the ratio of the spiked isotope to the natural isotope in the sample.
The primary idea underlying isotope dilution is that the spike and natural isotope will disperse themselves uniformly in the sample. The amount of spike added is adjusted so that the final ratio of spiked to natural isotope is easily detectable. Based on this ratio and the known amount of spike added, the total amount of the element in the sample may be determined.
Because the standard (isotopically enriched form of analyte) is added directly to the sample, isotope dilution is considered as an internal standardisation method. Furthermore, unlike standard analytical procedures that rely on signal intensity, isotope dilution makes use of signal ratios. Because of these advantages, the process of isotope dilution is regarded as one of the most accurate chemistry measuring procedures.
The basic principle behind isotope dilution can be explained as follows:
- Preparation of the Spike: A known amount of the element’s stable isotope is introduced to the sample. This isotope should have chemical properties similar to the element being studied but can be differentiated using analytical procedures.
- Mixing and Equilibration: The spike and the sample are thoroughly mixed to ensure that the stable isotope is evenly distributed throughout the sample. During this process, the spike “dilutes” the concentration of the element in the sample.
- Analysis: The mixture is then analyzed using a suitable analytical method (such as mass spectrometry or atomic absorption spectroscopy) to measure the concentration of both the natural and spiked isotopes.
- Calculation: The concentration of the element in the original sample can be calculated by comparing the ratio of the spiked isotope to the natural isotope in the examined mixture. Because the amount of the spike is known, this ratio can be used to calculate the initial concentration of the element in the sample.
Applications
This approach is especially beneficial for elements that are difficult to test directly or when there are interferences in the sample. Isotope dilution is frequently used in conjunction with mass spectrometry or other analytical techniques to achieve great precision and accuracy in elemental analysis. Isotope dilution is employed in nuclear reactor physics and other fields that demand exact measurements of isotope concentrations, in addition to analytical chemistry.