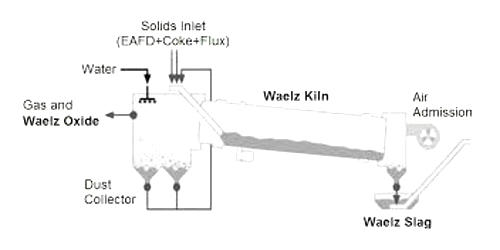
The Waelz process is one of the most efficient technologies, in terms of capacity and quality, able to recover nearly 90% of zinc content from such powders. The Waelz process is a method of recovering zinc and other relatively low boiling point metals from metallurgical waste (typically EAF flue dust) and other recycled materials using a rotary kiln (waelz kiln). Developed by German firms at the beginning of the 20th century, with the first operating plant in 1925.
The zinc enriched product is referred to as waelz oxide and the reduced zinc by-product as waelz slag. The material containing zinc (oxide, silicate, carbonate, zinc ferrite) must be pelletized with a carbon reductant. The Waelz process is applied on industrial scale in the metallurgy of zinc and it is designed to the enrichment of materials with low metal content to produce a concentrated zinc oxide (Waelz oxide).
History and description
The concept of using a rotary kiln for the recovery of Zinc by volatilization dates to at least 1888. A process was patented by Edward Dedolph in 1910. Subsequently, the Dedpolph patent was taken up and developed by Metallgesellschaft (Frankfurt) with Chemische Fabrik Griesheim-Elektron but without leading to a production scale ready process. In 1923 the Krupp Grusonwerk independently developed a process (1923), named the Waelz process; the two German firms later collaborated and improved the process marketing under the name Waelz-Gemeinschaft (German for Waelz association).
The carbon reductant must be coal with low volatiles content, only the carbon units which are not volatilized are useful for the zinc fuming reaction: ZnO(ore) + C(coal) -> Zn(gas) + CO(gas).
The volatile part of the coal is liberated at the very beginning of the kiln, with a little part of this thermal energy being valorized inside the kiln. Although Waelz technology has been around for over 80 years, the design and technology surrounding the basic kiln have improved throughout this time.
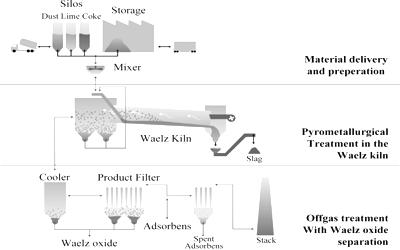
The process consists of treating zinc-containing material, in which zinc can be in the form zinc oxide, zinc silicate, zinc ferrite, zinc sulphide together with a carbon-containing reductant/fuel, within a rotary kiln at 1000°C to 1500°C. It is a process used for the extraction of zinc and lead from ores using a rotary kiln. Pellets preparation reduces dust formations, they are fed into a rotary kiln where the temperature is progressively increased up to 1100-1400 °C. The kiln feed material comprising zinc ‘waste’, fluxes, and reductant (coke) is typically pelletized before addition to the kiln. Zinc is still largely extracted from ores although around 40 percent of zinc is recycled from galvanized steels and other scrap metals. The chemical process involves the reduction of zinc compounds to elemental zinc (boiling point 907°C) which volatilizes, which oxidizes in the vapor phase to zinc oxide. The zinc oxide is collected from the kiln outlet exhaust by filters/electrostatic precipitators/settling chambers etc. Energy-efficient and clean, the Waelz Kiln technology is especially suited to the increasing demands of environmental laws being implemented globally.