Lime softening is a type of water softening treatment that involves the addition of limewater (calcium hydroxide) to reduce hardness (calcium and magnesium salt deposits) through precipitation. It is a water treatment procedure that removes calcium (Ca2+) and magnesium (Mg2+) ions from water to lessen its hardness.
By flocculation, the method is also excellent at removing a variety of bacteria and dissolved organic materials. Hard water contains large concentrations of these ions, which can cause scaling in pipes and appliances, impair the efficacy of soap and detergents, and cause various problems in industrial operations and home water usage.
History
Lime softening was first used to purify Thames River water in 1841. The process’s popularity grew as more advantages were revealed. Lime softening became increasingly popular in the early 1900s as industrial water usage increased. Lime softening produces soft water, which can be used more successfully for heat transfer and other industrial purposes in some instances.
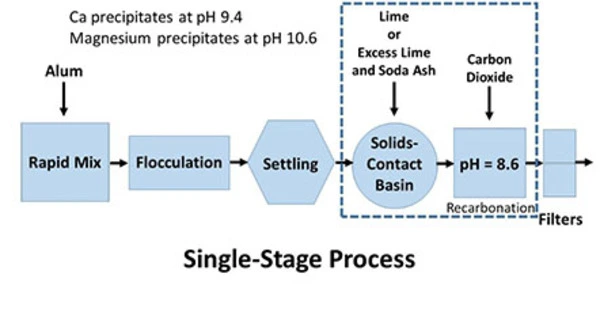
The lime softening process typically involves the following steps:
- Coagulation: Coagulants such as aluminum sulfate (alum) or ferric chloride are added to water to form tiny, sticky particles known as floc. These coagulants aid in the collection and binding of pollutants, making them easier to remove.
- Flocculation: Following coagulation, the water is gently stirred to enhance floc particle aggregation into larger, settleable clumps. This is known as flocculation, and it is normally done in a sedimentation basin.
- Sedimentation: The water is left to rest undisturbed in this step, and the larger floc particles sink to the basin’s bottom due to gravity. Most of the contaminants and suspended materials are removed during the sedimentation process.
- Softening: The softened water is then treated with lime (calcium hydroxide, Ca(OH)2). The lime reacts with the calcium and magnesium ions in the water to form insoluble precipitates, primarily calcium carbonate (CaCO3) and magnesium hydroxide (Mg(OH)2). These precipitates settle out of the water.
- Clarification: The water is subjected to further clarification to remove any remaining solids and precipitates. This can be achieved through additional settling or by using filters.
- pH Adjustment: Lime softening tends to raise the pH of the water. To ensure the final water quality meets regulatory standards and is not excessively alkaline, acid may be added to adjust the pH if necessary.
- Disinfection: Before the treated water is distributed for use, it is disinfected to kill any remaining microorganisms. Common disinfection methods include chlorination or ultraviolet (UV) disinfection.
The end result of the lime softening process is much softer water with lower amounts of calcium and magnesium ions. Because this treated water is less likely to create scaling in pipes and appliances, it is better suited for home and industrial use.
Lime softening is extensively utilized in municipal water treatment plants and industrial processes to remove water hardness. It is a proven approach for improving water quality by eliminating hardness minerals and other contaminants.