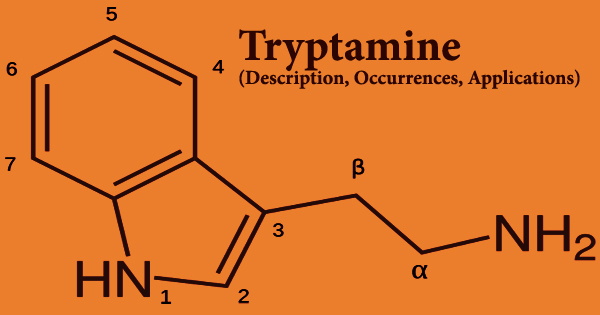
Decarboxylation of the amino acid tryptophan produces tryptamine, which is a monoamine alkaloid. An indole, fused benzene, and pyrrole ring, and a 2-aminoethyl group at the third carbon characterize the chemical structure. Tryptamine is present in fungi, plants, Amphibia, mammals, and microorganisms, among other things. Certain aminergic neuromodulators, such as melatonin, serotonin, bufotenin, and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin, and others, share tryptamine’s structure.
A number of mechanisms, notably monoamine oxidase, metabolize tryptamines, reducing some substances’ oral bioavailability. It has been found to activate trace amine-associated receptors expressed in mammalian brains and to control dopaminergic, serotonergic, and glutamatergic systems activity. The indole ring is the critical core of many complex natural compounds with drug discovery potential, as well as several synthetic and non-synthetic medicines based on the tryptamine skeleton.
Multiple tryptamine-derived migraine medications have been produced, and trace amine-associated receptors are being investigated as a possible therapeutic target for neuropsychiatric diseases. The chemical’s structure is similar to that of the neurotransmitter serotonin, which is found in well-known medications and hallucinogens. Because tryptamine is found in tiny levels in the mammalian brain, its importance as a psychedelic drug, neuromodulator, and neurotransmitter is well recognized.
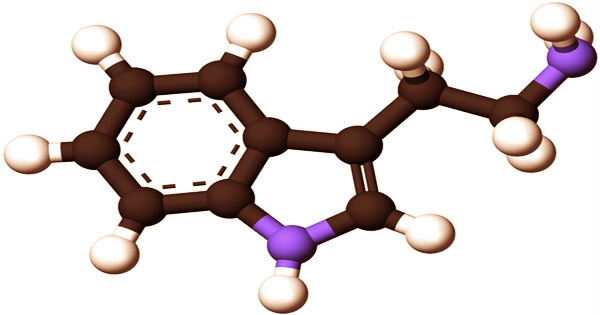
Mitragynine, a herbal supplement linked to seizures, and ergine, an alkaloid found in seeds of the morning glory family with a structure similar to LSD, are two ergoline-type tryptamines. Chronic tryptamine usage causes serotonergic neurotoxicity in animal models. In the mammalian brain, endogenous tryptamine levels are less than 100ng per gram of tissue. Trace amine levels have been shown to be increased in individuals with neuropsychiatric diseases such as bipolar depression and schizophrenia.
In one biochemical route, tryptamine functions as a promising phase for the plant hormone indole-3-acetic acid in modest quantities. N, N-dimethyltryptamine (DMT) is a tryptamine derivative that is a key component in the hallucinogenic effect of the “vine of the souls” brew. Humans and rats have relatively high levels of tryptamine in their guts and feces. In the gastrointestinal system, commensal bacteria such as Ruminococcus gnavus and Clostridium sporogenes produce the enzyme tryptophan decarboxylase, which assists in the conversion of dietary tryptophan to tryptamine.
Tryptamines are available as pills or powder and can be taken in a variety of ways. Indigenous Amazonian communities have historically utilized the drink as a medicinal therapy for a variety of physical ailments and addictions. The most frequent fungi that contain tryptamine derivatives are magic mushrooms. Serotonin and melatonin are produced through synthetic modifications of tryptamine; nevertheless, these routes do not occur naturally as the major mechanism for endogenous neurotransmitter production.
The desire to experience a new hallucinogen, the ease of availability owing to its legal status, the inexpensive cost, and the fact that they are undetectable by regular drug tests are all compelling reasons to take tryptamines. The major enzymes involved in tryptamine metabolism to generate indole-3-acetaldehyde are monoamine oxidases A and B, however, it’s unclear which isoform is unique to tryptamine breakdown. Small amounts of tryptamine are found in the mammalian brain, and it acts as a modulator or neurotransmitter by releasing serotonin agents. It’s also a serotonergic activity booster.
Tryptamine has been regarded as a trace neuromodulator capable of controlling the activity of neuronal cell responses without binding to the corresponding postsynaptic receptors in a few investigations. Analogs of tryptamine, which are generally created by synthetic modification, play an important role in humans owing to the introduction of physiologically active functions in its nucleus, which may induce changes in the mental and physical condition of the human brain.
Numerous neuroactive chemicals, ranging from anti-migraine medicines to hazardous substances, such as rizatriptan, sumatriptan, and zolmitriptan, are produced by substituting nitrogen and C-2 of the indole ring. Serotonin GPCRs are widely distributed along the colonic epithelium, and tryptamine generated by mutualistic bacteria in the human gut activates them. For a variety of reasons, a minimal quantity of tryptamine is necessary owing to its deaths and intoxication.
Information Sources: