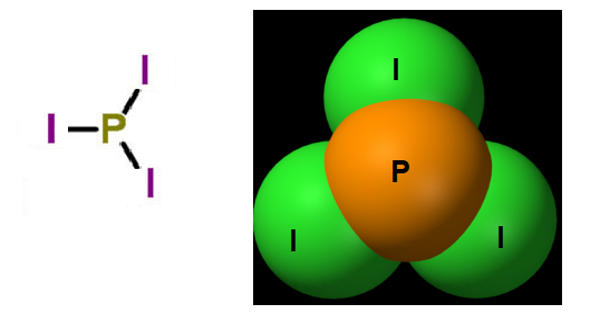
Phosphorus triiodide is a red solid unstable chemical compound with the formula PI3. It is an inorganic compound with the formula PI3. It is a trihalide of phosphorus that can be used as a deactivator and a deoxygenating agent. A red solid, it is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent.
Properties
Phosphorus triiodide a hexagonal red crystalline material is prepared by the direct action of iodine on white phosphorus in carbon disulfide. PI3 has a low dipole moment in carbon disulfide solution, because the P-I bond has almost no dipole. The P-I bond is also weak; PI3 is much less stable than PBr3 and PCl3, with a standard enthalpy of formation for PI3 of only −46 kJ/ mol (solid).
- Formula: PI3
- Formula weight: 411.687
- Class: iodide
- Colour: red
- Appearance: crystalline solid
- Melting point: 61°C
- Boiling point: 227°C
- Density: 4200 kg m-3

Reactions
Phosphorus triiodide reacts vigorously with water, producing phosphorous acid (H3PO3) and hydroiodic acid (HI), along with smaller amounts of phosphine and various P-P-containing compounds. Alcohols likewise form alkyl iodides, this providing the main use for PI3.
Phosphorus triiodide reacts violently with water forms phosphorus acid and hydrogen iodide. The chemical equation is given below.
PI3 + 3H2O → H3PO3 + 3HI
Preparation
The molecule of phosphorus triiodide is pyramidal in shape with very low polarity of the phosphorus iodine bond. The usual method or preparation is by the union of the elements, often by addition of iodine to a solution of white phosphorus in carbon disulfide:
P4 + 6 I2 → 4 PI3
Alternatively, PCl3 may be converted to PI3 by the action of hydrogen iodide or certain metal iodides. This is consistent with the observed zero dipole moment of the compound in carbon disulfide solution.
Uses
- Phosphorus triiodide is commonly used in the laboratory for the conversion of primary or secondary alcohols to alkyl iodides.
- It is used as a reagent for replacing hydroxyl groups by chlorine.
- The alcohol is frequently used as the solvent, on top of being the reactant. Often the PI3 is made in situ by the reaction of red phosphorus with iodine in the presence of the alcohol.
- It is used as a classic reagent for the conversion of aliphatic alcohols into iodides.
- PI3 is used in the synthesis of black phosphate crystal, which can be used as a semiconducting material for optoelectronic applications.
- It is used in the preparation of phosphoric acid by combining with water.
- It is also a source of triiodide that can be utilized as a charge carrier in electrolytes for dye sensitized solar cells.
Information Source: