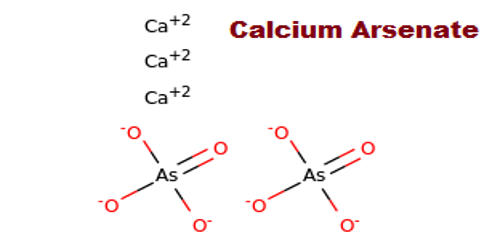
Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. It was registered for food use as an insecticide on various field and orchard crops. It is a pentavalent form of inorganic arsenic. A colorless solid, it was originally used as a pesticide and as a germicide. It normally exists as white crystals with no discernable odor. It is highly soluble in water, as compared with lead arsenate, which makes it more toxic.
Calcium arsenate contains 38% arsenic and is very slightly soluble in cold water and soluble in dilute acids. The minerals Rauenthalite Ca3(AsO4)2·10H2O and Phaunouxite Ca3(AsO4)2·11H2O are hydrates of calcium arsenate. The melting point is 1045 degrees C; the specific gravity is 3.62 and the molecular weight is 398.08.
Properties
- Compound Formula: As2Ca3O8
- Molecular Weight: 398.07
- Appearance: Colorless to white, odorless solid.
- Melting Point: N/A
- Boiling Point: N/A
- Density: 2.31 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 397.690283
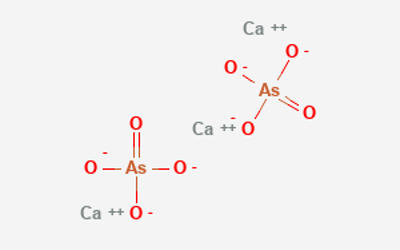
Fig: Calcium Arsenate Structure
Preparation
Calcium arsenate is commonly prepared from disodium hydrogen arsenate and calcium chloride:
2 Na2H[AsO4] + 3 CaCl2 → 4 NaCl + Ca3[AsO4]2 + 2 HCl
In the 1920s, it was made in large vats by mixing calcium oxide and arsenic oxide. In the United States, 1360 metric tons were produced in 1919, 4540 in 1920, and 7270 in 1922. The composition of commercially available calcium arsenate varies from manufacturer to manufacturer. A typical composition is 80-85% of Ca3(AsO4)2 a basic arsenate probably with a composition of 4CaO.As2O5 together with calcium hydroxide and calcium carbonate.
Use as a herbicide
It was registered as a foliar insecticide on various small fruits and berries and certain vegetable crops. It was once a common herbicide and insecticide. It was applied at a rate of 4.5 lb. active ingredient/acre. 38,000,000 kilograms were reported to be produced in 1942 alone, mainly for the protection of cotton crops. Its high toxicity led to the development of DDT. It was applied to the soil near plants for protection.
Regulation
Calcium arsenate use is now banned in the UK, and its use is strictly regulated in the United States. Its label states that it will “reduce and inhibit earthworm activity and survival” and is only recommended against serious earthworm infestations in places such as golf course greens. Calcium arsenate is moderately toxic to birds, slightly toxic to fish and moderately toxic to aquatic invertebrate species.