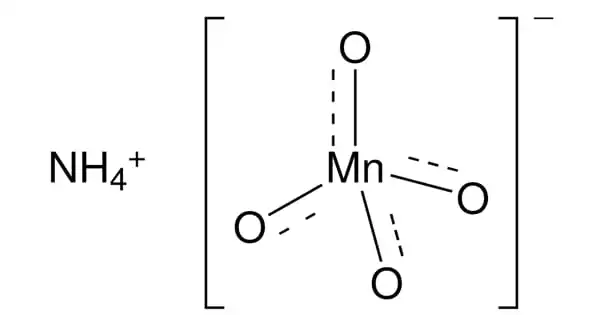
The chemical formula for ammonium permanganate is NH4MnO4, also known as NH3•HMnO4. It is a violet-brown or dark purple salt that is water soluble. It is a powerful oxidizing agent. It dissolves in water to form strong purple solutions, which evaporate to form prismatic purplish-black gleaming crystals. One of the most common chemicals used in the film and television industries to “age” items and set dressings is potassium permanganate. Potassium permanganate, being an oxidant, can serve as an antiseptic.
One of potassium permanganate’s most important industrial applications is as an oxidizing agent in the chemical synthesis of several key chemicals. In rural regions, it is used to remove iron and hydrogen sulfide from well and waste water. Aside from its use in water treatment, the other major application of potassium permanganate is as a reagent for the synthesis of organic compounds.
Preparation
Eilhard Mitscherlich invented ammonium permanganate in 1824 by reacting silver permanganate with an equivalent molar amount of ammonium chloride, sifting the silver chloride, and evaporating the water.
Potassium permanganate is commercially manufactured by combining a solution of KOH and powdered manganese oxide with oxidizing agents such as potassium chlorate. The mixture is boiled, evaporated, and the residue is cooked in iron pans until it acquires a pasty consistency.
AgMnO4 + NH4Cl → AgCl + NH4MnO4
It can also be prepared in a similar way from barium permanganate and ammonium sulfate.
Ba(MnO4)2 + (NH4)2SO4 → BaSO4 + 2 NH4MnO4

Properties
Because of the combination of the oxidizer permanganate anion and the reducing ammonium cation, ammonium permanganate is a strong oxidizer and a moderately strong explosive. Heat, shock, or friction can cause dry ammonium permanganate to detonate, and it can explode at temperatures exceeding 140 °F (60 °C).
- Appearance: Dark purple solid
- Crystal Structure: Orthorhombic
- Density: 2.703 g/cm3
- Melting Point: 240 °C
- Molar Mass: 158.034 g/mol
Ammonium permanganate decomposes explosively to manganese dioxide, nitrogen, and water:
2 NH4MnO4 → 2 MnO2 + N2 + 4 H2O
Ammonium permanganate decomposes slowly in storage even at normal temperatures. A sample stored for 3 months was only 96% pure, after 6 months it assumed color of iodine and had strong smell of nitrogen oxides. It emits toxic fumes when decomposed by heat.
Quaternary ammonium permanganate compounds can be prepared, such as tetrabutylammonium permanganate and benzyltriethylammonium permanganate.
Chemical Properties
Because potassium permanganate is a very strong oxidizing agent, it can be utilized as an oxidant in a wide range of chemical reactions. The oxidizing power of potassium permanganate can be shown by executing a redox reaction with it, in which the dark purple solution becomes colorless and eventually brown.
Uses
In qualitative analysis, potassium permanganate is utilized to get the permanganate value. In well water treatment, KMnO4 is also utilized as a regeneration chemical to remove hydrogen sulphide and iron. This substance is also used as a disinfectant to treat skin problems such as foot fungus infections and dermatitis.
Another notable application of potassium permanganate is in the treatment of bacterial infections. KMnO4 is also reported to be used in tanning leather and printing fabrics. This molecule can potentially be used as a bleaching agent, insecticide, and antiseptic.
Effects on Health
Potassium permanganate is an irritant to the eyes and skin when it is concentrated. It can react with a variety of reducing agents and organic materials, however it is combustible.
The antibacterial effect of KMnO4 is reliant on the oxidation of proteins in bacteria or tissues by this molecule. It leaves a stain on the skin or tissues. Because it destroys all organic materials through a destructive oxidation process, its application is limited to external uses exclusively.