Glass is an amorphous solid, which means it lacks the ordered internal structure of a crystal. Glass is created by cooling a mixture of materials so that it does not crystallize. Rubber, plastic, and gels are examples of amorphous solids. The fact that glass is an amorphous solid is what makes it so valuable in everyday life. It is used in a variety of applications, but it can still be improved to be more useful in a variety of settings.
Researchers have succeeded in developing a new type of super-stable, long-lasting glass with applications ranging from medicines to advanced digital screens to solar cell technology. The study demonstrates how combining multiple molecules, up to eight at a time, can result in a material that outperforms the best-known glass formers.
Researchers at the Chalmers University of Technology in Sweden have created a new type of super-stable, durable glass with potential applications ranging from medicines to advanced digital screens to solar cell technology.
By simply mixing many different molecules, we have suddenly opened up the possibility of creating new and improved glassy materials. Those who work with organic molecules are aware that using mixtures of two or three different molecules can aid in the formation of glass, but few would have predicted that the addition of more molecules, and this many, would produce such superior results.
Professor Christian Müller
A glass, also known as an ‘amorphous solid,’ is a material that lacks a long-range ordered structure and thus does not form a crystal. Crystalline materials, on the other hand, have a well-ordered and repeating pattern. The absence of crystals in a glass is what makes it useful.
The materials we commonly refer to as “glass” in everyday life are mostly silicon dioxide-based, but glass can be formed from a variety of materials. As a result, researchers are constantly looking for new ways to encourage different materials to form this amorphous state, which could lead to the development of new types of glass with improved properties and new applications. The new study, which was recently published in the scientific journal Science Advances, is a significant step forward in that search.
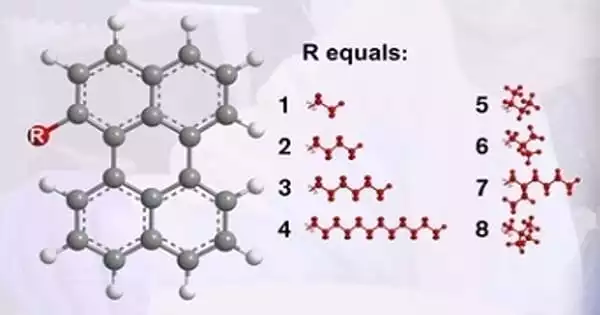
Sandra Hultmark, a doctoral student at the university’s Department of Chemistry and Chemical Engineering, stated that the glass product they created represented the best glass-forming ability that had been measured for organic material, inorganic material, and polymers. It outperforms the glass-forming ability of ordinary window glass, which is regarded as one of the best glass formers.

“By simply mixing many different molecules, we have suddenly opened up the possibility of creating new and improved glassy materials. Those who work with organic molecules are aware that using mixtures of two or three different molecules can aid in the formation of glass, but few would have predicted that the addition of more molecules, and this many, would produce such superior results”, According to Professor Christian Müller of Chalmers University’s Department of Chemistry and Chemical Engineering, who led the study’s research team.
The best result for any glass-forming material
Glass is formed when a liquid is cooled without crystallization, a process is known as vitrification. The use of two or three-molecule mixtures to promote glass formation is a well-established concept. However, little attention has been paid to the effect of mixing a large number of molecules on the ability to form a glass.
The researchers tested a mixture of up to eight different perylene molecules, each of which has a high fragility – a property related to how easily a material can form a glass. However, combining the many molecules resulted in a significant decrease in fragility, resulting in the formation of a very strong glass former with ultralow fragility.
“The fragility of the glass we created in the study is very low, representing the best glass-forming ability measured not only for any organic material but also for polymers and inorganic materials such as bulk metallic glasses. The results are even better than the glass-forming ability of ordinary window glass, which is one of the best glass formers we know of “Sandra Hultmark, a doctoral student at the Department of Chemistry and Chemical Engineering and the study’s lead author, explains.
Extending product life and saving resources
Display technologies such as OLED screens and renewable energy technologies such as organic solar cells are important applications for more stable organic glasses. “Glassy layers of light-emitting organic molecules are used to make OLEDs. If these were more stable, the durability of an OLED and, ultimately, the display would benefit “Sandra Hultmark elaborates.
Pharmaceuticals are another application that may benefit from more stable glasses. Amorphous drugs dissolve faster, allowing for faster absorption of the active ingredient after ingestion. As a result, many pharmaceuticals use glass-forming drug formations. It is critical for pharmaceuticals that the glassy material does not crystallize over time. The more stable the glassy drug, the longer the medicine’s shelf life.
“We could extend the lifespan of a large number of products by using more stable glasses or new glass-forming materials,” says Christian Müller, “offering savings in terms of both resources and economy.”