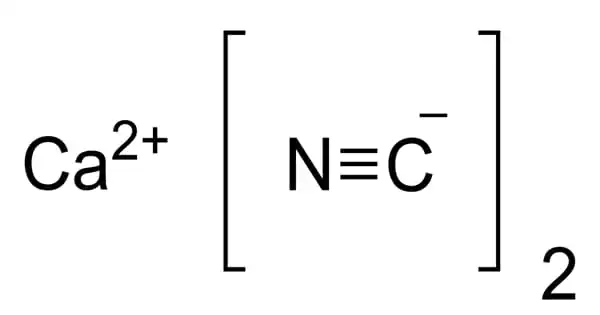Caesium cyanide (CsCN) is the hydrogen cyanide caesium salt. It’s a white solid that’s easily soluble in water, smells like bitter almonds, and has crystals that look like sugar. Caesium cyanide is extremely toxic and has chemical properties similar to potassium cyanide.
Cesium is the most electropositive and alkaline element, so it loses its single valence electron and forms ionic bonds with nearly all inorganic and organic anions more easily than any other element.
Cesium is a member of the periodic table’s alkali metal group, or Group 1. This element is gleaming and has a silvery-golden sheen to it. It is one of the most reactive metals on the planet; it is highly reactive and flammable. It is also one of only five metals that can exist as a liquid in a warm environment. Let’s find out more about this exciting, unpredictable element.
Properties
- Melting point: 350°C
- Density: 3.340
- Form: white cubic crystals
- Water Solubility: very soluble H2O
Production
Hydrogen cyanide reacts with caesium hydroxide giving caesium cyanide and water:
HCN + CsOH → CsCN + H2O.
It functions as both a basic salt and a reducing agent. It reacts with all acids to produce poisonous hydrogen cyanide gas. It can react violently with oxidizing agents, causing explosions when fusion occurs with metal chlorates, perchlorates, nitrates, or nitrites. Above 100°C, a mixture containing perchloryl fluoride may explode. A reaction with nitrite salts may result in an explosion. It has an incompatibility with iodine. It is responsible for the explosive decomposition of nitrogen trichloride.
Solid cesium reacts violently with water. When the two react, an aqueous solution of cesium hydroxide and hydrogen gas is formed. This exothermic reaction happens quickly and continues even after the solution becomes basic.
Cesium’s surface oxidizes and tarnishes when exposed to air. It also causes the formation of cesium superoxide on the metal’s surface, which has an orange-yellow color. This product is a stable salt that has been shown to be toxic to molecules in its surroundings. It plays a role in the pathogenesis of common diseases such as atherosclerosis and has the ability to damage DNA.
Fire Hazard
When exposed to acid, the highly flammable hydrogen cyanide gas is released. This material may volatilize as hydrogen cyanide when exposed to moisture. When heated to decomposition, it emits highly toxic cyanide and nitrogen oxide fumes. When combined with acids, it produces hydrogen cyanide gas. Strong oxidizers such as nitrates and chlorates, as well as nitrogen trichloride, perchloryl fluoride, sodium nitrate, acids, alkaloids, chloral hydrate, and iodine, react with it. Avoid coming into contact with acids.