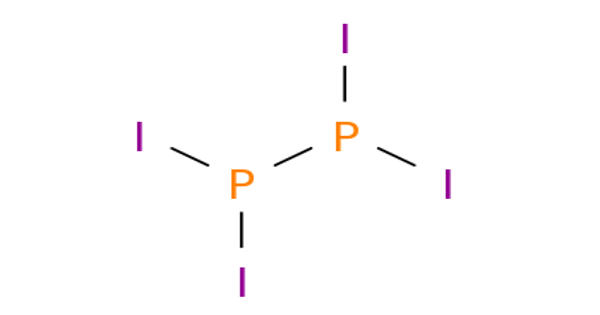
Potassium polonide is a chemical compound with the formula K2Po. It is a polonide, a set of very chemically stable compounds of polonium. Potassium is a chemical element with the symbol K and atomic number 19, while polonium is another chemical element with the symbol Po and atomic number 84. These elements can form compounds, but as of my last knowledge update in September 2021, I’m not aware of any compound called “Potassium Polonide.”
Properties
- Chemical formula: K2Po
- Molar mass: 287.18 g/mol
- Appearance: greyish
Characteristics
Potassium polonide is thermally more unstable and has stronger electron affinity than potassium telluride (K2Te). Potassium and polonium have very different properties and reactivity due to their positions in the periodic table. Potassium is an alkali metal, while polonium is a highly radioactive metalloid.
Production
Potassium polonide may be produced from a redox reaction between hydrogen polonide and potassium metal:
H2Po + 2 K → K2Po + H2
It may also be produced by heating potassium and polonium together at 300–400 °C. At higher temperature, this reaction may reverse.
Polonium (Po) is a highly radioactive chemical element with the atomic number 84. It’s a rare and extremely toxic metal. Polonium does not form stable compounds with potassium. It can, however, form compounds with other elements, such as oxygen and halogens.
Crystal structure
Like sodium polonide, potassium polonide has the antifluorite structure. Potassium is a chemical element with the symbol K and atomic number 19. It is a metal and is quite reactive, commonly found in nature as compounds like potassium chloride or potassium nitrate.