Acetylene, also known as Ethyne, is odorless but has a distinct garlic-like odor in commercial purity. It is very soluble in alcohol and almost miscible with ethane. It is the simplest and best-known member of the hydrocarbon sequence comprising one or more pairs of carbon atoms connected by triple bonds, known as the acetylenic series, or alkynes, with the formula C2H2. Acetylene is a flammable gas that is contained in gas cylinders under heat. Acetylene can react with copper, silver, and mercury to form acetylides, which can serve as ignition sources under some conditions. The flame will quickly flashback to the source of a leak. When exposed to fire or heat for an extended period of time, the containers can violently rupture and rocket.
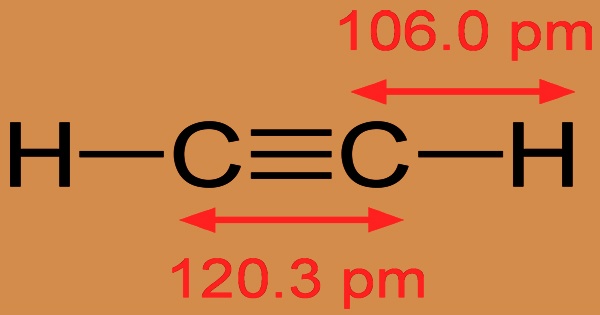
Acetylene is an alkyne, a gas molecular entity, and a terminal acetylenic compound. Acetylene becomes highly explosive when it is liquefied, compressed, heated, or combined with air. As a result, special care must be taken during the processing and handling of it. This colorless gas (lower hydrocarbons are naturally gaseous) is commonly used as a fuel and chemical building block. It is generally treated as a solution since it is unstable in its pure form. Although pure acetylene is odorless, impurities in commercial grades give it a distinct odor.
Brasses with less than 65 percent copper in the alloy, as well as some nickel alloys, are acetylene-compatible. Pure acetylene is a colorless gas with a good odor; but, when made from calcium carbide, it also contains traces of phosphine, which gives it a garlic-like odor. Solid oxidizers like chlorine, bromine pentafluoride, oxygen, oxygen difluoride, and nitrogen trifluoride, brass metal, calcium hypochlorite, heavy metals like copper, silver, mercury, and their salts, bromine, chlorine, iodine, fluorine, sodium hydride, caesium hydride, ozone, perchloric acid, and potassium are incompatible with acetylene.
Edmund Davy (1785-1857) discovered acetylene in 1836 while attempting to produce potassium metal from potassium carbide (K2C2). Marcel Morren successfully produced acetylene in 1859 by striking an electric arc with carbon electrodes in a hydrogen atmosphere. Carbon atoms were ripped away from the electrodes by the electric arc and bonded with hydrogen atoms to form acetylene molecules. This gas was named carbonized hydrogen by him. Since the two carbon atoms are bound together in a triple bond, acetylene is unsaturated as an alkyne. With CCH bond angles of 180°, the carbon-carbon triple bond binds all four atoms in a straight line.
Acetylene is a colorless, highly flammable compressed gas. When fresh, it has a slight ethereal odor; when polluted, it has a garlic-like odor. It’s easy to light and creates a sooty flame; the gas is lighter than air. Georges Claude and A. Hess discovered in 1897 that acetylene gas could be safely stored if it was dissolved in acetone. In 1905, Nils Dalen built long-burning, automatic marine and railroad signal lights using this new process. Dalen went on to create an acetylene torch for metal cutting and welding in 1906. The odors of divinyl sulfide and phosphine can be carried by commercially available acetylene gas. Acetylene can react with copper, silver, and mercury to form acetylides, which can serve as ignition sources under some conditions. Brasses contain compounds known as acetylides, which can serve as ignition sources. Brasses with less than 65 percent copper in the alloy, as well as some nickel alloys, are acetylene-compatible.

Acetylene has primarily been produced by partial combustion of methane since the 1950s. It’s a byproduct of the hydrocarbon cracking process that’s used to produce ethylene. In 1983, this process produced approximately 400,000 tonnes. As new methods for processing acetylene into useful plastics and chemicals were created, demand for acetylene increased. Demand peaked in the United States between 1965 and 1970, then sharply declined as new, lower-cost alternative conversion materials became available. Since the early 1980s, acetylene demand has been steadily growing at a rate of 2-4 percent per year.
Above about 760 mm Hg absolute, acetylene that has not been dissolved in acetone can deflagrate and become unstable. It has the ability to decompose explosively into hydrogen and carbon. Acetylene is 27.9 g per kg soluble in acetone at room temperature. The solubility of the same quantity of dimethylformamide (DMF) is 51 g. At 20.26 bar, the solubility of acetone and DMF increases to 689.0 and 628.0 g, respectively. In pressurized gas cylinders, these solvents are used. It’s also used in the organic chemicals industry to produce chloroethene (vinyl chloride), which is the starting material for polyvinyl chloride (PVC) and other vinyl compounds.
Acetylene produces a flame of over 3,600 K (3,330°C; 6,020°F) when combined with oxygen, releasing 11.8 kJ/g. The hottest burning common fuel gas is oxyacetylene. After dicyanoacetylene’s 5,260 K (4,990°C; 9,010°F) and cyanogen’s 4,798 K (4,525°C; 8,177°F), acetylene is the third-hottest natural chemical flame. Natural gas and petroleum were the primary sources of acetylene in Western Europe and Japan in 1991, while calcium carbide was the primary source in Eastern Europe and Japan. The simplest method produces acetylene gas and a calcium carbonate slurry, known as hydrated lime, by reacting calcium carbide with water. The following is an example of a chemical reaction
CaC2 + 2H2O → C2H2 + Ca(OH)2
Acetylene is commercially manufactured by pyrolysis of naphtha in a two-stage cracking process. End products include acetylene and ethylene. The naphtha feed rate can be adjusted to adjust the ratio of the two goods. Acetylene can also be manufactured from crude oil using a submerged-flame process. Acetylene was commonly used for illumination in the early twentieth century, including street lighting in some cities. Prior to the introduction of electric headlights, most early cars used carbide lamps. Pure acetylene was experimented with as an inhalation anesthetic in the 1920s.
Bacteria that live on acetylene have been described. Acetylene hydratase is an enzyme that catalyzes the hydration of acetylene to produce acetaldehyde:
C2H2 + H2O → CH3CHO
2CH4 → C2H2 + 3H2 is the chemical formula for converting methane into acetylene and hydrogen. The other gases are the byproducts of oxygen combustion. The acetylene is dissolved in a solvent such as water, anhydrous ammonia, chilled methanol, or acetone, or a mixture of solvents, depending on the process. When acetylene reacts with alkali metals, hydrogen gas is formed. It reacts violently with bromine. As acetylene is added to an aqueous solution of mercuric nitrate, it forms a sensitive acetylide.
Acetylene is most commonly used as a raw material in the manufacture of organic chemicals such as 1,4-butanediol, which is widely used in the production of polyurethane and polyester plastics. The fuel part in oxy-acetylene welding and metal cutting is the second most common usage. Acetylene black, which is used in some dry-cell batteries, and acetylenic alcohols, which are used in vitamin synthesis, are two commercially useful acetylene compounds. The heating of acetylene will result in highly exothermic polymerization reactions.
Acetylene, like other hydrocarbon gases, is an asphyxiant. Suffocation or asphyxiation by oxygen deprivation may result from exposure to its atmosphere. It is nontoxic at low concentrations but narcotic at high concentrations. However, due to its explosive properties, it is still only used as a local anesthetic in a small number of situations. The side effects of acetylene anesthesia have been identified as an increase in the amount of blood circulating, an increase in venous tonus, particularly in the abdominal cavity, an increase in blood pressure, and a displacement of blood from the inside to the peripheral blood vessels. Acetylene’s heating power can also be used for metal bending, straightening, shaping, hardening, softening, and strengthening.
Information Sources: