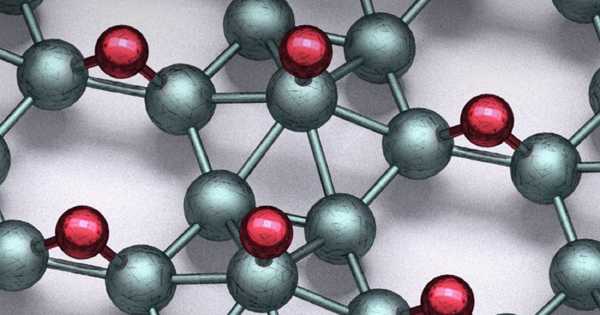
The new material preserves the electronic properties of borophene while providing new benefits. Northwestern University engineers have created a double layer of atomically flat borophene for the first time, defying boron’s natural tendency to form non-planar clusters beyond the single-atomic-layer limit.
Despite its promising electronic properties, borophene—a single-atom-layer-thick sheet of boron—is difficult to synthesize. Unlike its two-dimensional analog graphene, which can be peeled away from naturally layered graphite with something as simple as scotch tape, borophene cannot be peeled away from bulk boron. Instead, borophene must be grown directly onto a substrate.
If growing a single layer of atomically flat borophene was difficult, growing multiple layers of atomically flat borophene seemed impossible. Growing boron beyond single atomic layers results in clustering rather than planar films because bulk boron is not layered like graphite.
“When you try to grow a thicker layer, the boron wants to adopt its bulk structure,” said Mark C. Hersam, co-senior author of the study at Northwestern. “Thicker boron films, rather than remaining atomically flat, from particles and clusters. The key was to identify growth conditions that prevented cluster formation. We didn’t think you could go beyond one layer until now. We have now entered uncharted territory between the single atomic layer and the bulk, resulting in a new playground for exploration.”
The research will be published in the journal Nature Materials.
For the first time, Northwestern University engineers have created a double layer of atomically flat borophene, a feat that defies the natural tendency of boron to form non-planar clusters beyond the single-atomic-layer limit.
Hersam is the Walter P. Murphy Professor of Materials Science and Engineering at the McCormick School of Engineering and director of the Materials Research Science and Engineering Center. He also is a member of Northwestern’s International Institute for Nanotechnology and the Simpson Querrey Institute. Hersam co-led the work with Boris Yakobson, the Karl F. Hasselmann Chair in Engineering at Rice University.
Hersam and his colleagues discovered borophene for the first time five years ago. Borophene, which is stronger, lighter, and more flexible than graphene, has the potential to transform batteries, electronics, sensors, solar cells, and quantum computing. Although theoretical research predicted the possibility of a double layer of borophene, many researchers, including Hersam, were skeptical.
“It’s difficult to create new materials, even when theoretical work predicts their existence,” Hersam said. “Theory almost never tells you the synthetic conditions required to achieve that new structure.”
The substrate used to grow the material, Hersam’s team discovered, was the key to the proper conditions. Hersam and his colleagues grew borophene on a flat silver substrate for the study. When subjected to extremely high temperatures, the silver clumped together to form exceptionally flat, large terraces between clusters of atomic-scale steps.
“We saw a second layer forming when we grew borophene on these large, flat terraces,” Hersam explained. “Following that fortuitous observation, we purposefully directed our efforts in that direction. When we discovered the second layer, we weren’t looking for it. Many materials discoveries are made in this manner, but you must recognize the opportunity when you come across something unexpected.”
The double-layered material retained all of the desired electronic properties of borophene while adding new benefits. For example, the material could be made up of two atomic-layer-thick sheets bonded together with space in between and used for energy or chemical storage.
“Theoretical predictions indicate that bilayer borophene is a promising material for batteries,” Hersam said. “The space between the layers provides a place for lithium ions to accumulate.”
Hersam’s team hopes that this work will inspire other researchers to grow even thicker layers of borophene or to create double layers with different atomic geometries. “Diamonds, graphite, graphene, and carbon nanotubes are all based on the same element (carbon) but have different geometries,” Hersam explained. “Boron appears to have just as many possibilities as carbon, if not more. We think we’re still in the early stages of the two-dimensional boron saga.”