When you combine two or more materials, you form a mixture. In chemistry, a mixture is a substance that is made up of two or more simpler substances. These substances can be chemical elements or compounds. A mixture can be made of liquids, solids, or gases. The substances in a mixture can be separated using physical methods such as filtration, freezing, and distillation.
A mixture is not the same as a compound that is made of two or more atoms connected together. Mixtures have variable compositions, while compounds have a fixed, definite formula. For instance, a mixture of the gases hydrogen and nitrogen contains hydrogen and nitrogen, not the compound ammonia which is made of hydrogen and nitrogen atoms. Examples of Common Mixtures: Seawater – a mixture of water and various salts. Crude oil – a mixture of organic compounds – mainly hydrocarbons.
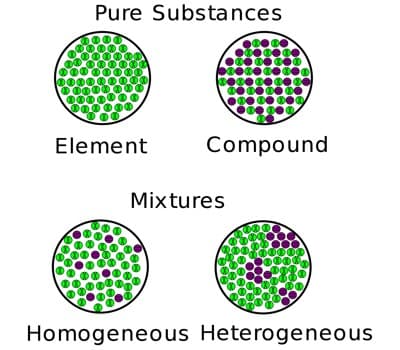
There are two categories of mixtures: homogeneous mixtures and heterogeneous mixtures. A mixture where the different parts can be distinguished easily is called heterogeneous, one where this is not the case is called homogeneous. A third form is called colloid. A colloid is a mixture where very small particles of one substance are evenly distributed throughout another substance. Examples of homogeneous mixtures include air, saline solution, most alloys, and bitumen. Examples of heterogeneous mixtures include sand, oil and water, and chicken noodle soup.
General properties of a mixture:
- The components of a mixture can be easily separated
- The components each keep their original properties
- The proportion of the components is variable
If one substance in a mixture dissolves in the other, it is called a solution. For example, if sugar is put in water it forms a mixture, then dissolves to make a solution. If it does not dissolve, it would be called a suspension.
Solids can be mixtures also. Alloys are mixtures. Many kinds of soil and rock are mixtures of different minerals. Thus, a mixture is made of two or more elements and/or compounds that are not chemically combined.