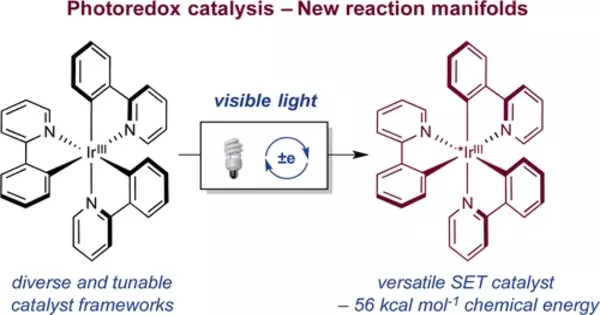
Photoredox catalysis is a type of catalytic reaction in which a photoredox catalyst is used to facilitate a chemical reaction by absorbing light and transferring an electron or an electron-hole. This type of catalysis is often used in organic chemistry, particularly for the synthesis of complex molecules and in the development of new chemical reactions. Photoredox catalysis can be used in combination with other catalytic methods, such as transition metal catalysis, to improve the efficiency and selectivity of chemical reactions.
It is a type of photochemistry that makes use of single-electron transfer. Transition-metal complexes, organic dyes, and semiconductors are the most common materials used as photoredox catalysts. While organic photoredox catalysts dominated in the 1990s and early 2000s, soluble transition-metal complexes are now more widely used. It is a type of chemical reaction that utilizes light energy to facilitate the transfer of electrons between molecules. In these reactions, a molecule called a photocatalyst absorbs light energy and uses it to transfer an electron to a substrate, thus triggering a chemical transformation.
Photoredox catalysis can be used in a variety of applications, such as organic synthesis and water splitting. It is a relatively new field of research and has been the subject of much recent interest due to its potential to provide new, more efficient, and sustainable ways of performing chemical reactions.
Photoredox catalysis is a relatively new and rapidly developing field, and many new applications and potential uses are currently being explored. It is becoming an increasingly popular approach in synthetic chemistry due to its high selectivity, mild reaction conditions, and compatibility with a wide range of substrates.
Photoredox catalysis has emerged as a powerful strategy for the activation of small molecules in organic chemistry in recent years. In general, these approaches rely on the ability of metal complexes and organic dyes to convert visible light into chemical energy via single-electron transfer with organic substrates, resulting in reactive intermediates. In this Perspective, we highlight photoredox catalysis’ unique ability to accelerate the development of completely new reaction mechanisms, with a focus on multicatalytic strategies that enable the formation of difficult carbon-carbon and carbon-heteroatom bonds.