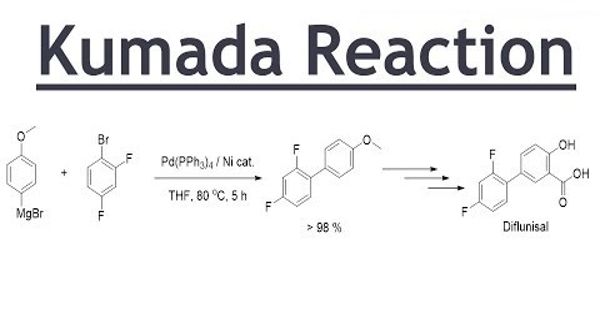
A Kumada coupling or Kumada-Corriu coupling is a cross-coupling reaction in organic chemistry between an alkyl or aryl Grignard reagent and an aryl or vinyl halocarbon catalyzed by nickel or palladium. In organic chemistry, the Kumada coupling is a type of cross-coupling reaction, useful for generating carbon-carbon bonds by the reaction of a Grignard reagent and an organic halide. It is an alternative for C – C bond formation and uses environmentally benign magnesium organometallics. The Kumada reaction of a Grignard reagent and an organic halide represents an alternative methodology for the functionalization of piperidines.
The Kumada coupling is a type of cross-coupling reaction, useful for generating carbon-carbon bonds by the reaction of a Grignard reagent and an organic halide. This reaction is relevant to organic synthesis because it gives access to styrene compounds.
The formation of the C–C bond is a major and important reaction in synthetic organic chemistry and frequently catalyzed by transition metal catalysts. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyls, aryl, or vinyl groups. The Kumada Coupling was the first Pd or Ni-catalyzed cross-coupling reaction, developed in 1972. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. Over the past three decades, transition‐metal‐catalyzed cross‐coupling reactions have emerged as one of the most important classes of C−C bond‐forming reactions. One of the oldest and most important transformations is the coupling of aryl halides with Grignard reagents. This chemistry has been extensively studied using Pd2 and Ni3 catalysis since its first discovery by Kumada and Corriu in 1972.
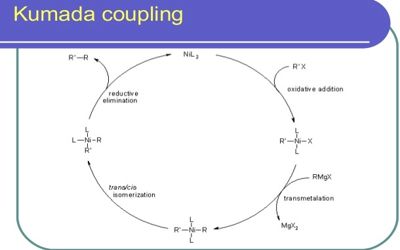
The reaction is notable for being among the first reported catalytic cross-coupling methods. It is yet another C–C cross-coupling reaction catalyzed by nickel or palladium and involves the coupling of the aryl Grignard reagents with the aryl halide, sulfamate, and ether substrates that yield the corresponding biaryl compounds.
Despite the subsequent development of alternative reactions (Suzuki, Sonogashira, Stille, Hiyama, Negishi), the Kumada coupling continues to be employed in many synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. The advantage of this reaction is the direct coupling of Grignard reagents, which avoids additional reaction steps such as the conversion of Grignard reagents to zinc compounds for the starting materials in the Negishi Coupling.
Information Source: